
via@ub_Sanath

Hester acquires technology from ICAR – IVRI for Classical Swine Fever and Sheep Pox Vaccines
https://www.deshgujarat.com/2021/04/08/ ... -vaccines/
Gujarat headquartered Hester has signed two agreements with ICAR-IVRI (Indian Council of Agricultural Research – Indian Veterinary Research Institute),for acquiring technologies for the production and commercialisation of the following vaccines:
1. Classical Swine Fever Vaccine
2. Sheep Pox Vaccine
These vaccines are the first that have been developed within the country by using locally isolated strains, a step towards making India self-sufficient, Atmanirbhar, for the country’s requirement of Classical Swine Fever Vaccine and Sheep Pox Vaccine.
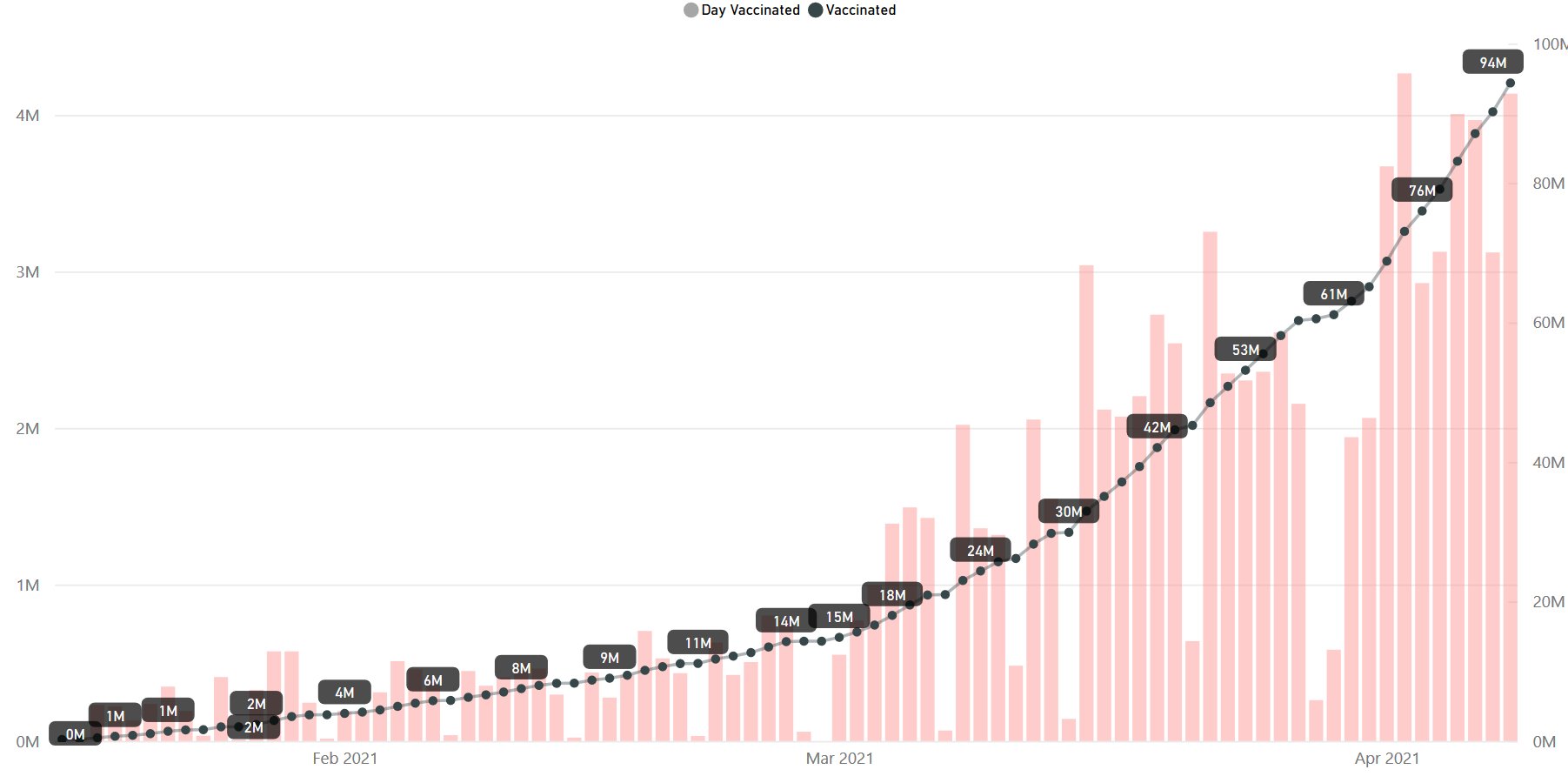
Day 83: India #vaccination update on 09-Apr at 7 AM as per
@MoHFW_INDIA
Total Doses: 9,43,34,262 Up-pointing triangle 36,91,511
It is reasonable protocol given that all the vaccines are in EUA mode right now. Every national government still has the ultimate responsibility to its public and cannot simply respond to any later problem with ‘they trialed elsewhere, we thought it was ok’. Pfizer was asked to do trials but they declined . AZ did trials locally , and Sputnik V is also doing it now. Rules are same for everyone.Uttam wrote:J&J is about to start a clinical trial of its COVID vaccine in India.
What is need for a local clinical trial? If this is to check the efficacy of the vaccine on Indian ethnicity, I am sure we can find enough data from the US and other places on ethnic Indians getting the J&J vaccine. Any vaccine expert on the forum who can shed some light, please.
That makes sense. I am just wondering if we can find some "safe" "short-cuts" to the process. I know there is no vaccine shortage yet. But given the rate at which we are vaccinating and the vaccine production rate, the shortage will hit us soon. I hope Sputinik V, if safe, gets approved soon. Zycov-D is also in phase 3 trial. With an abbreviated trial of J&J, we will have one more vaccine.Suraj wrote: It is reasonable protocol given that all the vaccines are in EUA mode right now. Every national government still has the ultimate responsibility to its public and cannot simply respond to any later problem with ‘they trialed elsewhere, we thought it was ok’. Pfizer was asked to do trials but they declined . AZ did trials locally , and Sputnik V is also doing it now. Rules are same for everyone.
We have a pretty good pipeline of vaccines in various stages of trials, in addition to ongoing capacity expansions for both Covishield and Covaxin. Dr Reddy's are investing in Sputnik V supply.Uttam wrote:That makes sense. I am just wondering if we can find some "safe" "short-cuts" to the process. I know there is no vaccine shortage yet. But given the rate at which we are vaccinating and the vaccine production rate, the shortage will hit us soon. I hope Sputinik V, if safe, gets approved soon. Zycov-D is also in phase 3 trial. With an abbreviated trial of J&J, we will have one more vaccine.Suraj wrote: It is reasonable protocol given that all the vaccines are in EUA mode right now. Every national government still has the ultimate responsibility to its public and cannot simply respond to any later problem with ‘they trialed elsewhere, we thought it was ok’. Pfizer was asked to do trials but they declined . AZ did trials locally , and Sputnik V is also doing it now. Rules are same for everyone.
Any news about whether or not the local production of Sputnik V has already started? I saw a lot of news about local producers signing contracts but none about whether they have started production.
Pharmaceutical firm Bharat Biotech would expand its manufacturing capacity to meet the demand for Covaxin, CNBC TV18 reported on March 25.
Covaxin is one of the two COVID-19 vaccines approved so far by the Indian health regulator. The anti-coronavirus shot is currently being manufactured at the Hyderabad-based plant of Bharat Biotech.
The company would soon launch a new production facility in Malur Kolar district of Karnataka, while ramping up the existing manufacturing capacity in Hyderabad, the channel reported citing sources.
The production at the Malur Kolar plant would begin shortly after it is officially launched. The manufacturing capacity at the plant would be raised five-fold till July-end, the report added.

https://www.livemint.com/news/india/bha ... 01891.htmlBharat Biotech targets 70 crore Covaxin doses
Currently, Bharat Biotech is manufacturing 40 lakh doses through its manufacturing plant at Hyderabad. However, the company’s chairman and managing director, Krishna Ella had indicated that the firm planned to make 70 crore doses of Covaxin in 2021.
The company, this month, had bagged orders of 2 crore doses of Covaxin from the Ministry of Health and Family Welfare.
Apart from using company-owned sites, the government is considering other proposals. For instance, the Maharashtra government wrote to the central government proposing the use of Mumbai-based Haffkine Biopharmaceutical Corporation for manufacturing Covaxin.
Why do you expect vaccination to have an impact on the number of cases? We have nowhere near the coverage at this point. Maybe death rate, but not absolute number of cases. There are no lockdowns so far so it is not surprising that the rate of growth is steeper.saip wrote:Mid Sep 20 we had 1,017,000 active cases. Then the no kept dropping to 135k by Mid. Feb 21. Now it is crossing the million mark. What is surprising is the steepness of the rise of this wave. And the vaccinations are not stopping it. We seem to be testing over a million everyday (yday we had 1.3 mil tests) What are we testing? Is it rapid anti-gen test or is it RT-PCR? Are they giving false positives?
It seems this company is hiding something. What do the Pharma Gurus think?An expert panel of India's drug regulator on Friday sought additional data from drugmaker Dr.Reddy's Laboratories on its Sputnik V COVID-19 vaccine trial, in its second such request after an initial evaluation in February.
The committee asked the company to submit a comparative analysis of late-stage immunogenicity data from both its Indian studies and an ongoing Russian study, as well as data on serious adverse events and positive cases reported till date.
Dr.Reddy's has been conducting small clinical trials with Sputnik V in India under a deal with Russia's wealth fund. Several Indian companies have signed deals to produce and supply over half a billion doses of the vaccine.
Why not? The vaccination program is targeted at high risk groups like frontline workers and the elderly so shouldnt that have an impact on numbers? if not then what is the point of the program we might as well focus on just the coverage.Raja wrote:
Why do you expect vaccination to have an impact on the number of cases? We have nowhere near the coverage at this point. Maybe death rate, but not absolute number of cases. There are no lockdowns so far so it is not surprising that the rate of growth is steeper.
Looks like all "adenovirus vector vaccines" are having issues.European regulators are reviewing side effects of Johnson & Johnson's single-dose Covid-19 shot, after a handful of cases of rare blood clots were reported among its recipients.
Overall, the vaccine prevented moderate to severe Covid-19 by 66.1 percent 28 days after the shot, but this rose to 85.4 percent when considering only severe disease.
What makes it most striking was that the good results against severe disease held up in both South Africa and Brazil, where concerning variants were dominant during the trial period.
Overall, the vaccine prevented moderate to severe Covid-19 by 66.1 percent 28 days after the shot, but this rose to 85.4 percent when considering only severe disease.
What makes it most striking was that the good results against severe disease held up in both South Africa and Brazil, where concerning variants were dominant during the trial period.
In late February, the US pharma giant said that at least one case of anaphylaxis (severe allergic reaction) had been reported -- and such reactions have also been recorded for other Covid-19 vaccines in rare instances.
The US Centers for Disease Control and Prevention (CDC) has advised people not to take the Johnson & Johnson shot if they have any history of severe allergic reactions.
J&J's shot is known as an "adenovirus vector vaccine" and the company previously produced a European Union-approved Ebola vaccine using the same technology.
Oxford-AstraZeneca and Sputnik's shots are both adenovirus vector vaccines, too.
They all use double-stranded DNA molecules to carry genetic instructions, rather than single-stranded RNA used by Pfizer and Moderna.
This is where the vaccine maitri programme helps - by exporting whatever we can at the same priority as producing for home use, we have earned some goodwill around the world. Now if the US continues with its dog-in-the-manger attitude of eating everything in sight and hoarding the rest (dogs usually don't hoard, but the US is a special caseg.sarkar wrote:https://news.yahoo.com/india-coronaviru ... 14438.html
India coronavirus: Can its vaccine producers meet demand?
Shruti Menon, April 7, 2021
Why is capacity 'stressed'?
Two vaccine producers in India have raised concerns about their ability to meet their production targets. The largest of these, SII - which produces Novavax and AstraZeneca vaccines - has warned of raw material shortages affecting production. Its chief executive, Mr Poonawalla, attributed this to US export bans on specific items needed to make vaccines, such as specialised bags and filters. The firm said it has also faced difficulties importing cell culture media, single-use tubing and specialised chemicals from the US. "The sharing of these... raw materials is going to become a critical limiting factor — nobody has been able to address this so far," said Mr Poonawalla. The SII had written to the Indian government in March asking it to intervene to ensure the uninterrupted manufacture and supply of vaccines globally.
Another Indian manufacturer, Biological E, which is producing the Johnson & Johnson vaccine, has also raised concerns about possible shortages affecting vaccine production. Mahima Datla, the company's chief executive, recently said US suppliers were "reluctant to commit that they will stick to their delivery timelines".
.....
Gautam
US as the sole super power does not bow to pressure and does not care what the others think. There are always two standards, one for the US and another for the rest of the world. India is looking at the vaccine as a means to further its soft power and help many nations with which we have little connection. It also wants to gain recognition with larger nations. US is looking at this opportunity as a means to control the rest and also to make billions of dollars for its pharma industry.Two different view points all together.arshyam wrote: This is where the vaccine maitri programme helps - by exporting whatever we can at the same priority as producing for home use, we have earned some goodwill around the world. Now if the US continues with its dog-in-the-manger attitude of eating everything in sight and hoarding the rest (dogs usually don't hoard, but the US is a special case), pressure can be brought to bear not just by us, but by a whole host of other countries who can raise a stink in the UN. Maybe some gora Europeans would also join in given their vaccine programmes are in a shambles, which would make a big difference to "global" public opinion. So this public disclosure of supply constraints is a good idea. Sustained hoarding that impacts production meant for a large part of the world could lead to a serious image problem for the amrikis if that happens. Yes, the US can still ignore the UN and continue doing what it wants to, but it will come at a high diplomatic cost. Unlike Trump, I don't see a Biden admin trying to antagonize the world too much due to their globalist pretentions. As for us, we can further leverage this attitude of the US down the line when trying to increase our influence in some countries at the expense of the US.
Net net, the idiots barking about Modi unnecessarily exporting vaccines don't know what they are talking about. As in most things done by the Modi govt, there is some strategy behind it.
Elderly are vaccinated because they are at high risk of dying from covid. Not because they are at a higher risk of contacting the disease. We don't have current cases broken down by age to make even a pseudo comparison.SandeepA wrote:
Why not? The vaccination program is targeted at high risk groups like frontline workers and the elderly so shouldnt that have an impact on numbers? if not then what is the point of the program we might as well focus on just the coverage.
Not getting this - why was Gujrat given so many doses?chetak wrote:
Well, the original expiry was set at 6 months. It is now 9 months for Covishield. I think GoI made a serious error in judgment thinking that India was done with Covid when the new case numbers were continuously falling for a few months from the previous peak. It did not anticipate numbers could do a quick u-turn and rise sharply due to change in season or any other factors.Suraj wrote:The vaccine export strategy isn't hard to parse. SII started production in December, and GoI started stockpiling some of it, but not all of it. It must be remembered that vaccines are perishable. It's not like kharif and rabi crops in FCI.
Sputnik V has done what oxford and BB has failed to do. They've arranged licensed production agreements with various indian vaccine makers in the country.Russian Direct Investment Fund has tied up manufacturing of over 850 million doses of Sputnik V in India, including agreements with Hetero Biopharma, Gland Pharma, Virchow Biotech, Panacea Biotech and Stelis Biopharma. Around 426 million doses are expected to be earmarked for use in the country.
Your justification that exports were done due to fear of expiry doesn't hold. We are clearly supply constrained at the moment. The error in judgment is to think that we didn't need all the doses produced. I am OK with exporting token numbers of doses but not huge numbers as of now.Suraj wrote:What action on the GoI's part demonstrates error in judgment ? The original vaccination plan made it clear they would first vaccinate HCWs, then the high risk groups working down from the highest risk groups to lower ones. That's exactly what has happened so far.
SII has been producing in excess of domestic consumption since late last year. This report from November states they've manufactured 40 million doses back then:a_bharat wrote:Your justification that exports were done due to fear of expiry doesn't hold. We are clearly supply constrained at the moment. The error in judgment is to think that we didn't need all the doses produced. I am OK with exporting token numbers of doses but not huge numbers as of now.Suraj wrote:What action on the GoI's part demonstrates error in judgment ? The original vaccination plan made it clear they would first vaccinate HCWs, then the high risk groups working down from the highest risk groups to lower ones. That's exactly what has happened so far.
On Thursday, Serum Institute of India said it has made 40 million doses of AstraZeneca's potential Covid-19 vaccine, and would soon begin making Novavax's rival shot, as they both seek regulatory approval, news agency Reuters reported.
Serum Institute of India, however, declined to comment on whether the 40 million doses of the AstraZeneca vaccine were meant for global supply or only for India, the Reuters report said.
By all means make credible arguments, but you have not quoted any independently verifiable reference material. You're simply making an assertion. You can choose to believe anything you wish, but it does not make me wrong.The export is happening to ensure that the precious vaccine, which has a life span of six months, does not expire. The current stock will start expiring from March.
Approx 65-70 million, plus domestic supplies of 110 million, of which almost 100 million have been actually used. The 110 million number is listed in the state-wise data posted earlier. The main unknown is how much has the government stockpiled. That can be determined by knowing monthly SII+BB production but this data is not clear.Kashi wrote:How many doses have we donated/exported in total, including the GAVI/CoVax programme?