It's been 11 months since the Wuhan virus happened. Are you still saying that all this happened just because everyone wanted to go against Trump?chanakyaa wrote:Now that the desired outcome has been achieved in a completely fair and transparent elections, time to release the magic potion.
Wuhan Coronavirus Resource Thread
Re: Wuhan Coronavirus Resource Thread
Re: Wuhan Coronavirus Resource Thread
He is talking about the timing of the announcement. Trump has been in loggerheads with the pharma companies for a while now over pricing.
Re: Wuhan Coronavirus Resource Thread
Not at all, Jay sir. CV is real, the damage caused by CV is real and lives impacted globally by the virus, sadly, is real. No doubt about that. What is not clear, and I doubt if it will ever be clear, is the timing of the virus, the true origins of the virus, and how it transmitted across borders (why certain countries were affected more or less than others etc.), and timing of the release of vaccine announcements. Worst yet, if we don't know why, you can't control the future outbreaks. Pfizer and BioNtech announced back in July about the availability of vaccine as early as October. To be fair, they never really committed to a specific date, so the timing of past weekend is good as their original claim; plus the testing can be wild card.
Re: Wuhan Coronavirus Resource Thread
Wow. That is great. I really like Atul Gawande and his ideas on how to fix the health care system in the US.Amber G. wrote:Sharing a recent picture of Dr. Atul Gwande's (who will be on Covid task force) parents - who immigrated to USA just around the time I did. Both have been serving rural Ohio as well respected doctors for last many decades. [img...]https://pbs.twimg.com/media/C32mIpRUcAA ... =4096x4096[/img]
Re: Wuhan Coronavirus Resource Thread
https://www.nytimes.com/2020/11/09/heal ... fizer.html
Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective
Pfizer announced positive early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed.
By Katie Thomas, David Gelles and Carl Zimmer
The drug maker Pfizer announced on Monday that an early analysis of its coronavirus vaccine trial suggested the vaccine was robustly effective in preventing Covid-19, a promising development as the world has waited anxiously for any positive news about a pandemic that has killed more than 1.2 million people.
Pfizer, which developed the vaccine with the German drugmaker BioNTech, released only sparse details from its clinical trial, based on the first formal review of the data by an outside panel of experts.
The company said that the analysis found that the vaccine was more than 90 percent effective in preventing the disease among trial volunteers who had no evidence of prior coronavirus infection. If the results hold up, that level of protection would put it on par with highly effective childhood vaccines for diseases such as measles. No serious safety concerns have been observed, the company said.
Pfizer plans to ask the Food and Drug Administration for emergency authorization of the two-dose vaccine later this month, after it has collected the recommended two months of safety data. By the end of the year it will have manufactured enough doses to immunize 15 million to 20 million people, company executives have said.
“This is a historical moment,” said Kathrin Jansen, a senior vice president and the head of vaccine research and development at Pfizer. “This was a devastating situation, a pandemic, and we have embarked on a path and a goal that nobody ever has achieved — to come up with a vaccine within a year.”
The news comes just days after Joseph R. Biden Jr. clinched a victory over President Trump in the presidential election. Mr. Trump had repeatedly hinted a vaccine would be ready before Election Day, Nov. 3. This fall, Pfizer’s chief executive, Dr. Albert Bourla, frequently claimed that the company could have some indication of whether the vaccine worked by October, something that did not come to p ass.
....
___________________________________________________________________________________________________________
https://www.bbc.com/news/world-asia-china-54883383
Coronavirus: China Sinovac vaccine trial halted in Brazil
The Brazilian clinical trial for a high-profile Chinese Covid-19 vaccine has been suspended after health authorities reported a "severe adverse" incident.
Brazilian health regulator Anvisa said it took place on 29 October, but did not give further details.
The Sinovac vaccine is one of several vaccines in final-stage testing.
But China has already been using it to immunise thousands of people in an emergency use programme.
There was no immediate response from Sinovac.
On Monday Anvisa said it had "ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident".
It did not reveal what happened, nor where it took place. Late-stage trials for the Sinovac vaccine are also being conducted in Indonesia and Turkey, but neither of these countries have announced a suspension.
Dimas Covas, the head of Butantan, the medical research institute conducting the Brazilian trial, told local media that the trial's suspension was related to a death.
But he insisted that the death was not related to the vaccine, reported Reuters news agency.
Butantan has said it would hold a news conference on Tuesday at 11am local time (1400 GMT).
,,,,,
Gautam
Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective
Pfizer announced positive early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed.
By Katie Thomas, David Gelles and Carl Zimmer
The drug maker Pfizer announced on Monday that an early analysis of its coronavirus vaccine trial suggested the vaccine was robustly effective in preventing Covid-19, a promising development as the world has waited anxiously for any positive news about a pandemic that has killed more than 1.2 million people.
Pfizer, which developed the vaccine with the German drugmaker BioNTech, released only sparse details from its clinical trial, based on the first formal review of the data by an outside panel of experts.
The company said that the analysis found that the vaccine was more than 90 percent effective in preventing the disease among trial volunteers who had no evidence of prior coronavirus infection. If the results hold up, that level of protection would put it on par with highly effective childhood vaccines for diseases such as measles. No serious safety concerns have been observed, the company said.
Pfizer plans to ask the Food and Drug Administration for emergency authorization of the two-dose vaccine later this month, after it has collected the recommended two months of safety data. By the end of the year it will have manufactured enough doses to immunize 15 million to 20 million people, company executives have said.
“This is a historical moment,” said Kathrin Jansen, a senior vice president and the head of vaccine research and development at Pfizer. “This was a devastating situation, a pandemic, and we have embarked on a path and a goal that nobody ever has achieved — to come up with a vaccine within a year.”
The news comes just days after Joseph R. Biden Jr. clinched a victory over President Trump in the presidential election. Mr. Trump had repeatedly hinted a vaccine would be ready before Election Day, Nov. 3. This fall, Pfizer’s chief executive, Dr. Albert Bourla, frequently claimed that the company could have some indication of whether the vaccine worked by October, something that did not come to p ass.
....
___________________________________________________________________________________________________________
https://www.bbc.com/news/world-asia-china-54883383
Coronavirus: China Sinovac vaccine trial halted in Brazil
The Brazilian clinical trial for a high-profile Chinese Covid-19 vaccine has been suspended after health authorities reported a "severe adverse" incident.
Brazilian health regulator Anvisa said it took place on 29 October, but did not give further details.
The Sinovac vaccine is one of several vaccines in final-stage testing.
But China has already been using it to immunise thousands of people in an emergency use programme.
There was no immediate response from Sinovac.
On Monday Anvisa said it had "ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident".
It did not reveal what happened, nor where it took place. Late-stage trials for the Sinovac vaccine are also being conducted in Indonesia and Turkey, but neither of these countries have announced a suspension.
Dimas Covas, the head of Butantan, the medical research institute conducting the Brazilian trial, told local media that the trial's suspension was related to a death.
But he insisted that the death was not related to the vaccine, reported Reuters news agency.
Butantan has said it would hold a news conference on Tuesday at 11am local time (1400 GMT).
,,,,,
Gautam
Re: Wuhan Coronavirus Resource Thread
The fear around these parts (Maharashtra) is totally gone. Testing seems to be sporadic, the follow up from government agencies for home quarantined folks seems to be on the wane, and the cases seem to be rising steadily in my immediate vicinity, as compared to the October numbers. I am using the arogya setu app to keep up with the numbers around me.
Re: Wuhan Coronavirus Resource Thread
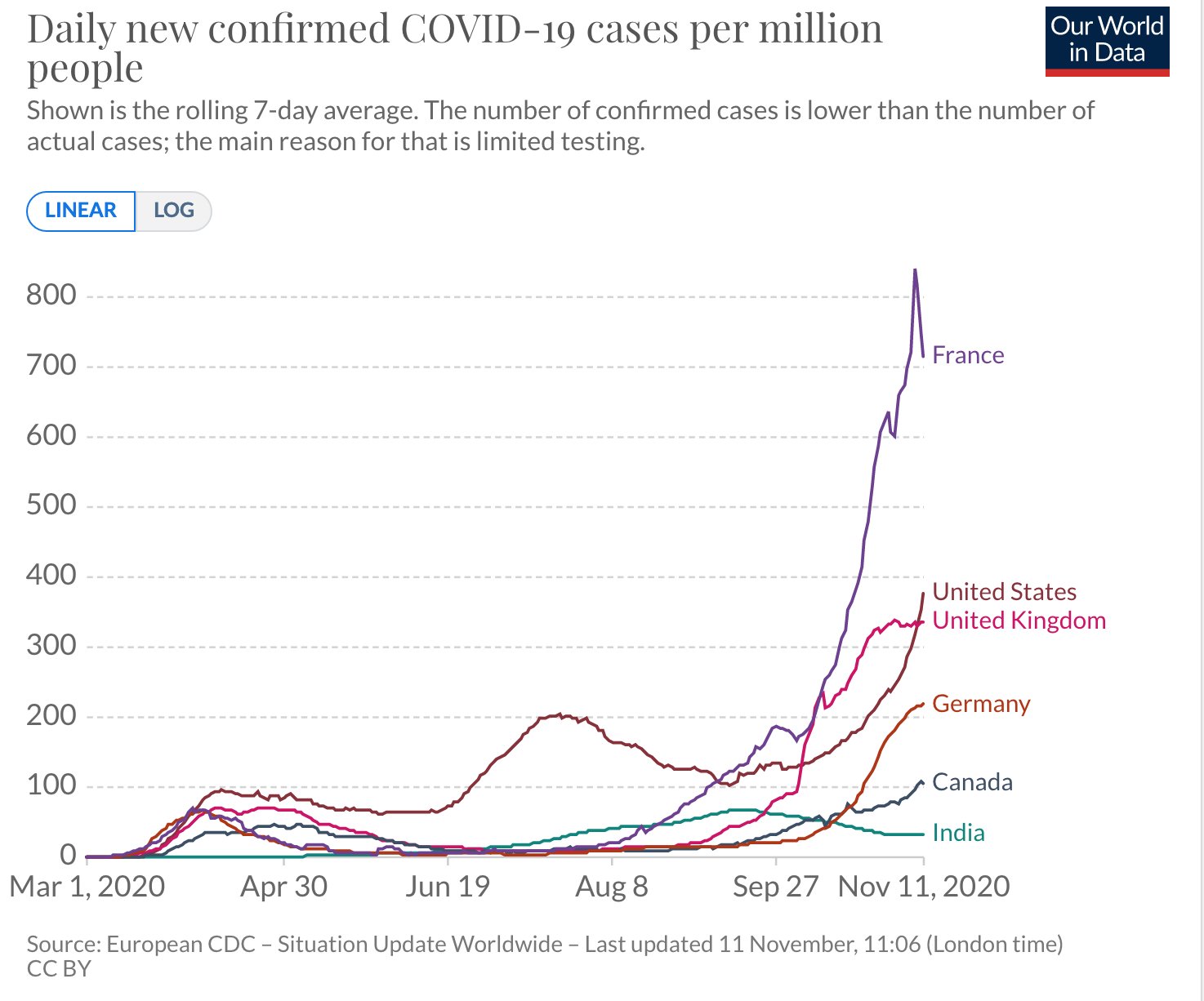
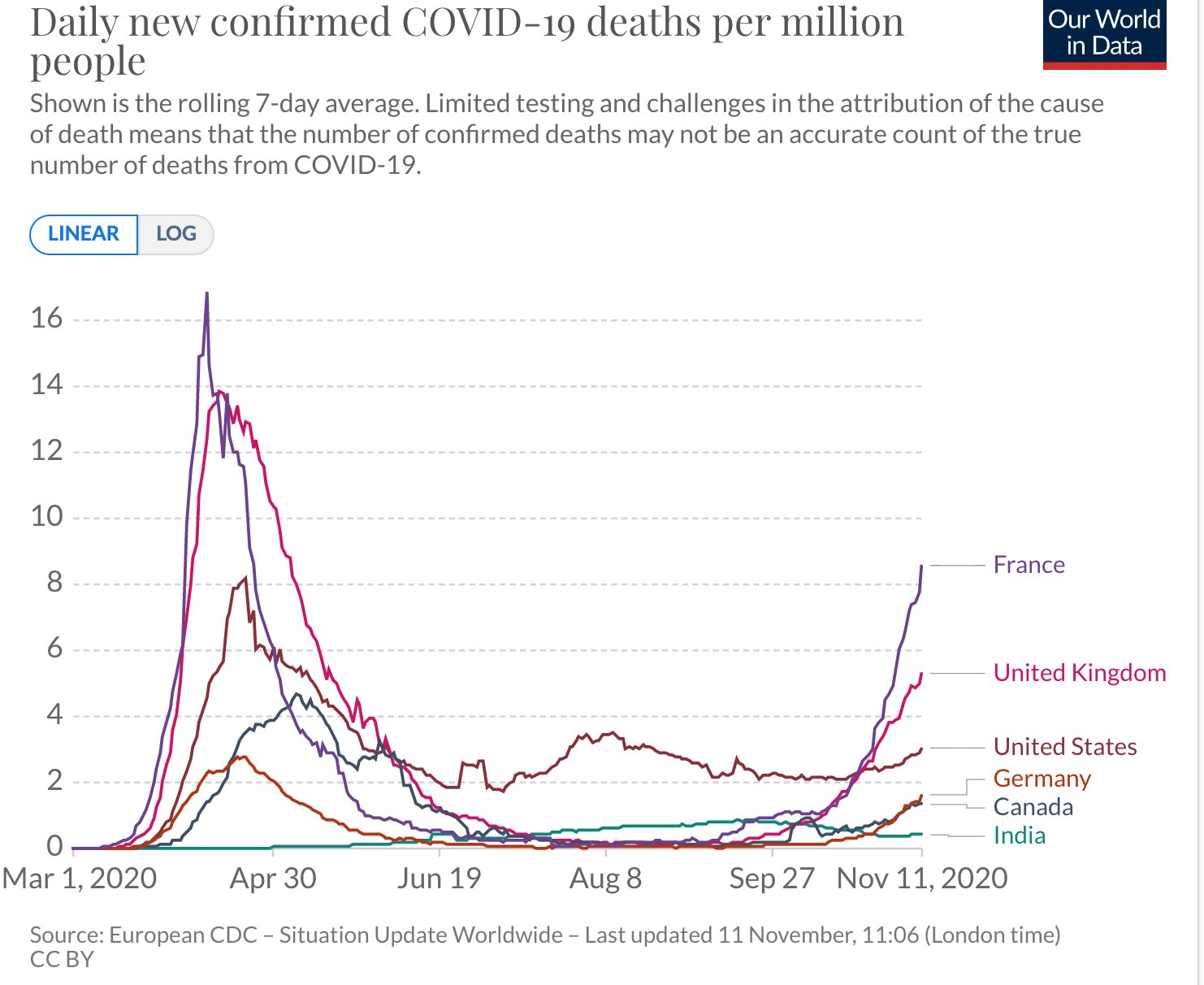
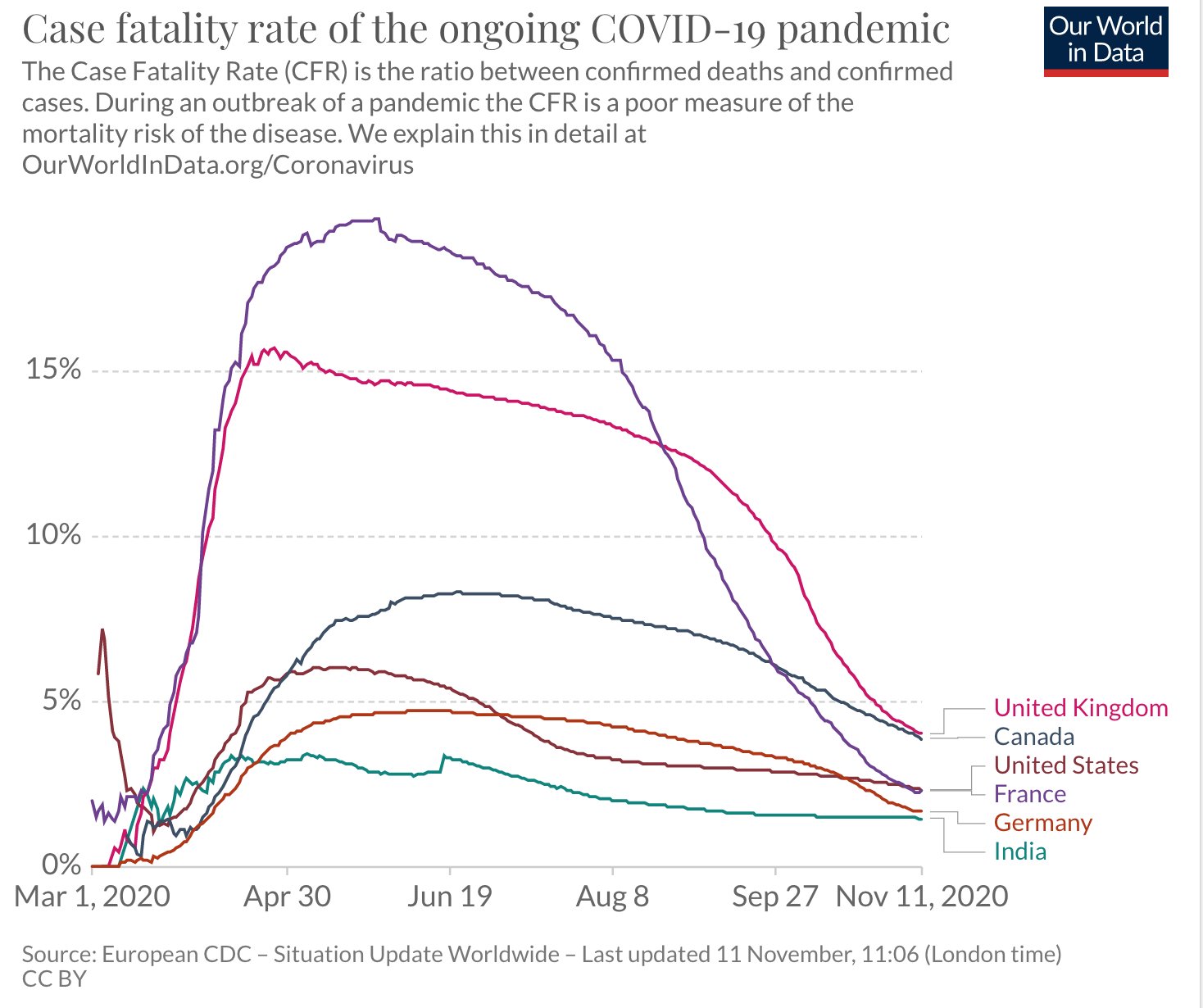
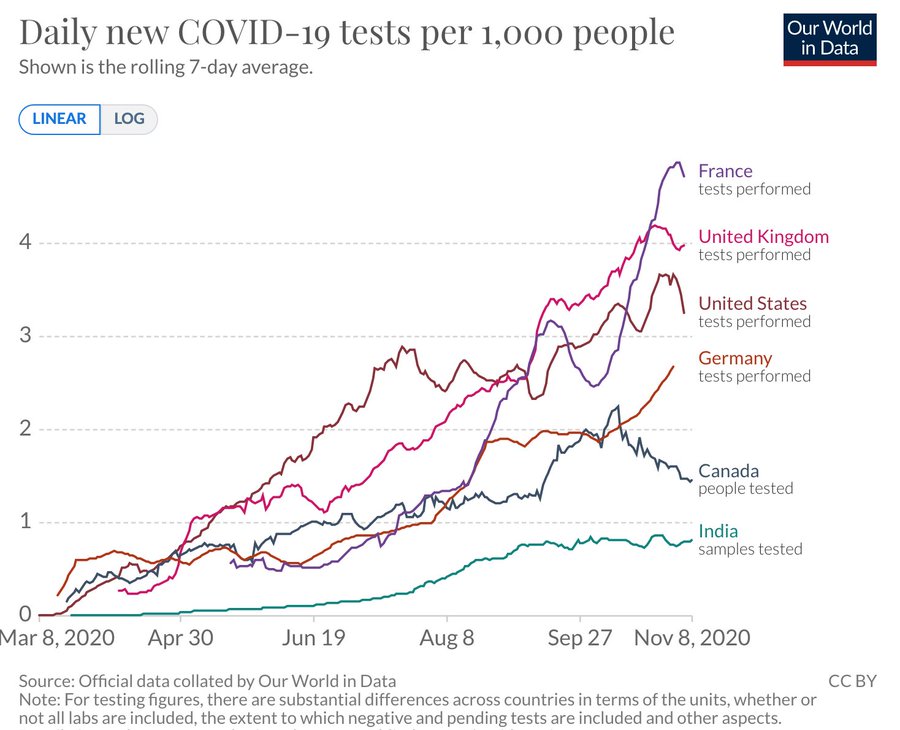
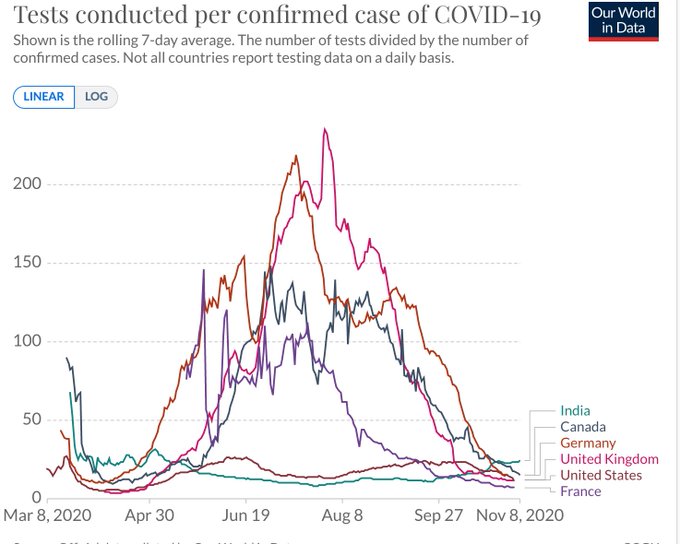
Sharing some graphs: about this pandemic as it is really exploding dangerously in my neighborhood and in other parts of the world.
The entire world ought to have the same goal: cases of COVID-19 need to go to zero. Only if we end the pandemic everywhere can we end the pandemic anywhere.
I have chosen a few countries of my interest - USA, Canada, France and Europe and India. The graphs give indication of the progress being made in various parts.
All data is normalized with cases / million to make comparison more meaningful.
Six graphs (see all the comments) including confirmed cases and confirmed deaths are produced using https://ourworldindata.org/ and European CDC data.
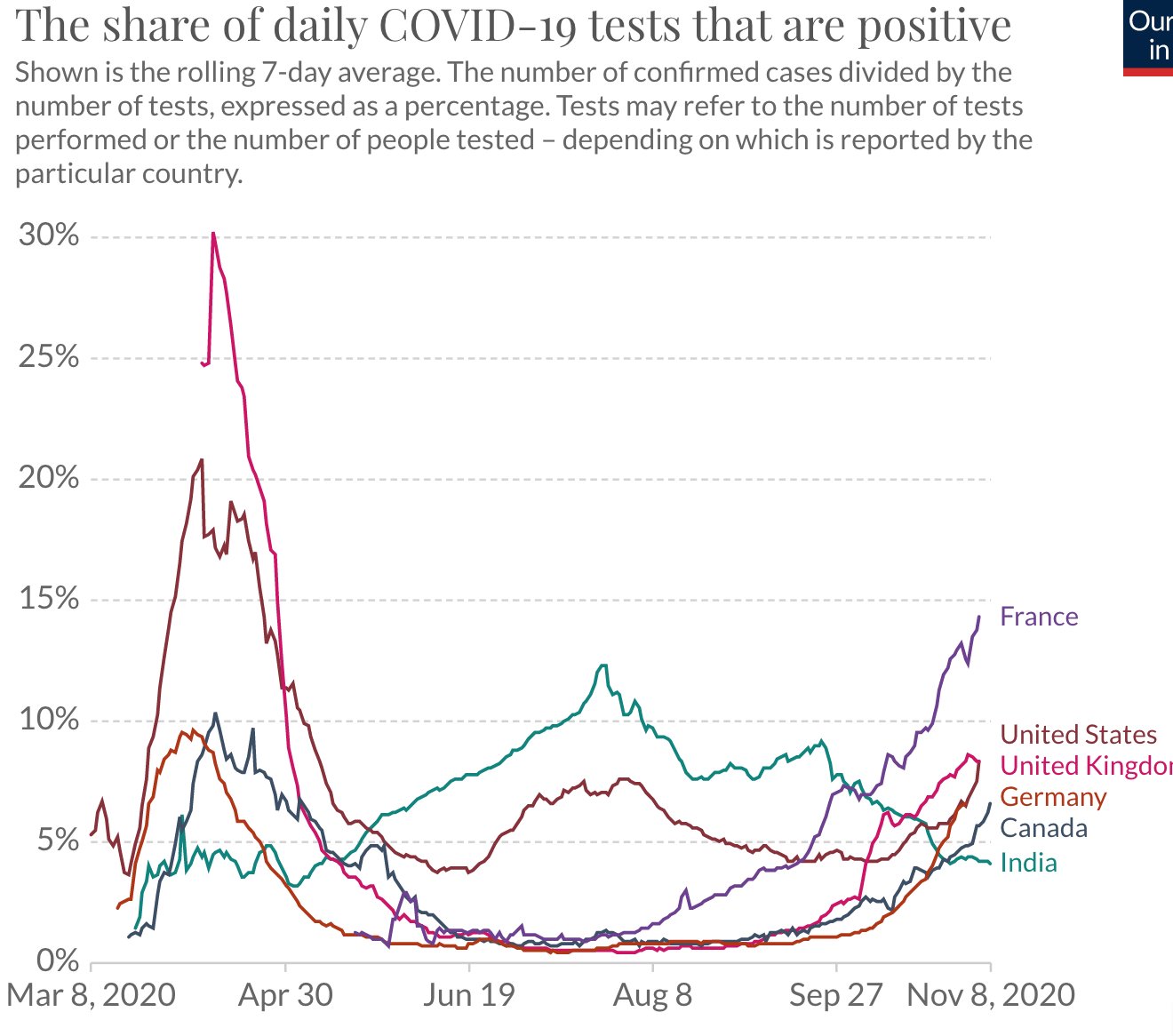
The data on confirmed cases only becomes meaningful when it can be interpreted in light of how much a country is testing. This is why there are other graphs. (Particularly interesting is the metric ‘share of positive tests’ – which shows you the share of tests that are confirming a test. This rate should ideally be very low. If it is high a country is likely not identifying a large share of cases).
I am particularly hopeful. India has done excellent work and the current projection is projecting that the rate will dwindle to almost zero by the end of February 2021. In the USA the incoming leadership has appointed world class scientists for its covid task force and I really hope that we start listening to science again. Recent news about vaccines is also very encouraging.
The entire world ought to have the same goal: cases of COVID-19 need to go to zero. Only if we end the pandemic everywhere can we end the pandemic anywhere.
I have chosen a few countries of my interest - USA, Canada, France and Europe and India. The graphs give indication of the progress being made in various parts.
All data is normalized with cases / million to make comparison more meaningful.
Six graphs (see all the comments) including confirmed cases and confirmed deaths are produced using https://ourworldindata.org/ and European CDC data.
The data on confirmed cases only becomes meaningful when it can be interpreted in light of how much a country is testing. This is why there are other graphs. (Particularly interesting is the metric ‘share of positive tests’ – which shows you the share of tests that are confirming a test. This rate should ideally be very low. If it is high a country is likely not identifying a large share of cases).
I am particularly hopeful. India has done excellent work and the current projection is projecting that the rate will dwindle to almost zero by the end of February 2021. In the USA the incoming leadership has appointed world class scientists for its covid task force and I really hope that we start listening to science again. Recent news about vaccines is also very encouraging.






Re: Wuhan Coronavirus Resource Thread
Yes, this is very encouraging. Be ready... there are some other very familiar names which are going to be announced.. including in areas like energy etc.Vayutuvan wrote:Wow. That is great. I really like Atul Gawande and his ideas on how to fix the health care system in the US.Amber G. wrote:Sharing a recent picture of Dr. Atul Gwande's (who will be on Covid task force) parents - who immigrated to USA just around the time I did. Both have been serving rural Ohio as well respected doctors for last many decades. [img...]https://pbs.twimg.com/media/C32mIpRUcAA ... =4096x4096[/img]
Hopefully pretty soon the disgusting era of Trump will go away .. admin will start listening to science again instead of enabling those despicable goons who issue death threats against us scientists.
Added later: Biden's chief of staff choice is quite impressive. Ron Klain ( Ebola "czar" - was highly respected professional by everyone)
Re: Wuhan Coronavirus Resource Thread
Thanks for posting this data Amber G.
France's case increase trend was expected after summer vacations & school reopenings (called the rentrée, which is actually the start of a brand new year of planning and execution for businesses, govt and politics, 01Jan is just a holiday!) which would see a flurry of activity and therefore create more opportunities for the virus to spread.
But the scale/accleration of the case count was a real surprise for everyone. Hospitals are already running near full in ICU capacity, prompting the Govt to impose a new phase of lockdown, though not as tough as the one before. Its good to see the case count trend dropping. Curiously, France has NOT adopted an aggressive testing policy at any point of time, though lots of test are being done, it is not seen as a tool to contain the spread much less eliminate it.
I suspect the govt doesn't want to see hospitals overrun by finding max no of cases. With the health mgmt resources they have and their high degree of saturation may be its a tacit decision to let infected people come fwd to get tested when their symptoms manifest clearly and will then be taken into required care. But this strategy is a double edged sword and has a clear down side. Asymptomatic spread will be high, and even symptomatic cases will be spreaders for some days until they go get tested and found positive and then isolated.
Just goes to show how difficult it is for Govt.s even when they take it very seriously, to predict/model and take the right measures at the right time contain the virus. Do the best you can and the rest is "Ram bharose".
Biden has hung his coat on changing the pandemic response and controlling it, which of course needs to be done, but given Trump's inaction so far and this will continue until Jan 20th, its quite probable that the situation in the US will be highly dangerous and near impossible to control for Biden & team. Americans or any people for that matter, don't deserve such a mess.
I remain a bit sceptical about virus efficiency claims, because there is so much $$$ at stake and its so tempting for big Pharma to overstate the +s and gloss over the -s. Vaccine is no miracle solution even if it works very well due to the time it takes to produce, deliver and verify results on actual populations re: immunity response intensity and lastingness.
Hope things will stay on the encouraging trend for India through out the festival season into the new year.
France's case increase trend was expected after summer vacations & school reopenings (called the rentrée, which is actually the start of a brand new year of planning and execution for businesses, govt and politics, 01Jan is just a holiday!) which would see a flurry of activity and therefore create more opportunities for the virus to spread.
But the scale/accleration of the case count was a real surprise for everyone. Hospitals are already running near full in ICU capacity, prompting the Govt to impose a new phase of lockdown, though not as tough as the one before. Its good to see the case count trend dropping. Curiously, France has NOT adopted an aggressive testing policy at any point of time, though lots of test are being done, it is not seen as a tool to contain the spread much less eliminate it.
I suspect the govt doesn't want to see hospitals overrun by finding max no of cases. With the health mgmt resources they have and their high degree of saturation may be its a tacit decision to let infected people come fwd to get tested when their symptoms manifest clearly and will then be taken into required care. But this strategy is a double edged sword and has a clear down side. Asymptomatic spread will be high, and even symptomatic cases will be spreaders for some days until they go get tested and found positive and then isolated.
Just goes to show how difficult it is for Govt.s even when they take it very seriously, to predict/model and take the right measures at the right time contain the virus. Do the best you can and the rest is "Ram bharose".
Biden has hung his coat on changing the pandemic response and controlling it, which of course needs to be done, but given Trump's inaction so far and this will continue until Jan 20th, its quite probable that the situation in the US will be highly dangerous and near impossible to control for Biden & team. Americans or any people for that matter, don't deserve such a mess.
I remain a bit sceptical about virus efficiency claims, because there is so much $$$ at stake and its so tempting for big Pharma to overstate the +s and gloss over the -s. Vaccine is no miracle solution even if it works very well due to the time it takes to produce, deliver and verify results on actual populations re: immunity response intensity and lastingness.
Hope things will stay on the encouraging trend for India through out the festival season into the new year.
Re: Wuhan Coronavirus Resource Thread
Today Filthy India has less number of active cases (489294) than TX (855503), CA (503132) and FL (606325).
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
Vaccine from Pfizer has been ordered by several hospital chains in the US. They will have them in December for doctors and nurses.
Re: Wuhan Coronavirus Resource Thread
any news on Oxford vaccine?
Re: Wuhan Coronavirus Resource Thread
Mort, Whats the impact on all those COVID test Kit makers once vaccines are viable?
Re: Wuhan Coronavirus Resource Thread
I still think there is a link between Nipah virus in Kerala in 2017 and this Wuhan Virus. Sadly am not a biology major to make the connections.
Just gut instinct.
Just gut instinct.
Re: Wuhan Coronavirus Resource Thread
^^Let us hope not. Though both are traced to bats, Nipha has extremely high moratality rate (over 75% vs 1.5 for COVID19 in India)
Re: Wuhan Coronavirus Resource Thread
^^^ To put it mildly, with genome sequencing, and having world class experts who know such things the "origin", spread etc have been/or being studied extremely well.. sure there is lot of unknowns but a few facts we know scientifically very well..including how the current virus is being evolved/getting mutated etc. India (including some IIT's, IISc and other places) have technology and expertise to do/understand/analyze/ sequencing.
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
Some of these new vaccines for COVID-19 are mRNA based and need cold storage. The Pfizer-BioNTech vaccine requires -80C temp for bulk storage. I'm not a biologist, but my wife is a molecular biologist and was telling me the Pfizer vaccine is probably 60-70% effective as opposed to the 90% some 7 days after the second dose. Data from phase-3 of the AstraZeneca/Oxford vaccine should be coming out in the next couple of weeks. From what I understand it is a viral-vector vaccine which is more effective and requires storage at -8C. Additionally, it is cheaper per dose.ramana wrote:Mort, Whats the impact on all those COVID test Kit makers once vaccines are viable?
The need for COVID test kits will continue well into next year.
Re: Wuhan Coronavirus Resource Thread
TDS folks are still smarting from 'filthy India' comment, hain?!!! it was about air quality. we all know that air quality is no great shakes in bhaarat, especially in Dehli - lair of the elites at of the elite.saip wrote:Today Filthy India has less number of active cases (489294) than TX (855503), CA (503132) and FL (606325).
Re: Wuhan Coronavirus Resource Thread
Pfizer's vaccine announcement seems to be timed wrt its CEO selling shares than Trump's re-election chances.
Re: Wuhan Coronavirus Resource Thread
Some shenannigans was going on. They only needed to have 32 pts catch COVID to publish their preliminary results, but they waited until they got more than twice the pts.Cyrano wrote:Pfizer's vaccine announcement seems to be timed wrt its CEO selling shares than Trump's re-election chances.
Re: Wuhan Coronavirus Resource Thread
https://news.yahoo.com/why-covid-19-kil ... 11975.html
'Breakthrough finding' reveals why certain Covid-19 patients die
'Breakthrough finding' reveals why certain Covid-19 patients die
In an international study in Science, 10 percent of nearly 1,000 Covid-19 patients who developed life-threatening pneumonia had antibodies that disable key immune system proteins called interferons. These antibodies — known as autoantibodies, because they attack the body itself — weren't found at all in 663 people with mild or asymptomatic Covid-19 infections. Only four of 1,227 healthy patients had the autoantibodies. The study was led by the Covid Human Genetic Effort, which includes 200 research centers in 40 countries.
"This is one of the most important things we've learned about the immune system since the start of the pandemic," said Dr. Eric Topol, executive vice president for research at Scripps Research in San Diego, who wasn't involved in the new study. "This is a breakthrough finding."
In a second Science study by the same team, the authors found that an additional 3.5 percent of critically ill patients had mutations in genes that control the interferons involved in fighting viruses. Given that the body has 500 to 600 of those genes, it's possible that researchers will find more mutations, said Qian Zhang, lead author of the second study.
Interferons serve as the body's first line of defense against infection, sounding the alarm and activating an army of virus-fighting genes, said virologist Angela Rasmussen, an associate research scientist at the Center for Infection and Immunity at Columbia University's Mailman School of Public Health.
"Interferons are like a fire alarm and a sprinkler system all in one," said Rasmussen, who wasn't involved in the new studies.
Lab studies show that interferons are suppressed in some people with Covid-19, perhaps by the virus itself.
Re: Wuhan Coronavirus Resource Thread
FWIW (Okay for those who are scientifically curious, not allelic to science, and not into conspiracy theories etc  ) WRT to news on the Pfizer+BioNTech Vaccines:
) WRT to news on the Pfizer+BioNTech Vaccines:
There is a nice writeup by K. Vijay Raghavan (Principal Scientific Adviser to the Government of India) and some others in DST) I will put if I can find a nice link. His comments "Good news, in sum, but challenges remain. Patience yet, and no let-up on prudent behaviour"
There is paper by Harvard's Marc Lipsitch and UF's Natalie Dean to understand vaccine efficacy etc. (see below).
The announcement and details about the vaccine is here:
PFIZER AND BIONTECH ANNOUNCE VACCINE CANDIDATE AGAINST COVID-19 ACHIEVED SUCCESS IN FIRST INTERIM ANALYSIS FROM PHASE 3 STUDY
https://science.sciencemag.org/content/370/6518/763
There is a nice writeup by K. Vijay Raghavan (Principal Scientific Adviser to the Government of India) and some others in DST) I will put if I can find a nice link. His comments "Good news, in sum, but challenges remain. Patience yet, and no let-up on prudent behaviour"
There is paper by Harvard's Marc Lipsitch and UF's Natalie Dean to understand vaccine efficacy etc. (see below).
The announcement and details about the vaccine is here:
PFIZER AND BIONTECH ANNOUNCE VACCINE CANDIDATE AGAINST COVID-19 ACHIEVED SUCCESS IN FIRST INTERIM ANALYSIS FROM PHASE 3 STUDY
>>> Following are quotes from > Dean's writeup: about the big news with apparent high efficacy (>90%) based on 94 confirmed COVID-19 cases at their interim analysis.:Highlights:
[*]- Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis
- Analysis evaluated 94 confirmed cases of COVID-19 in trial participants
- Study enrolled 43,538 participants, with 42% having diverse backgrounds, and no serious safety concerns have been observed; Safety and additional efficacy data continue to be collected
- Submission for Emergency Use Authorization (EUA) to the U.S. Food and Drug Administration (FDA) planned for soon after the required safety milestone is achieved, which is currently expected to occur in the third week of November
- Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints
Added later: Here is the link for the above paper (may require subscription)- How to interpret this news. Briefly: "Celebrate, but let the process play out over time as intended."
- When the vaccine is highly effective, we need less data to see it. While trials are planned for 150+ total events, this is what we need for a 60% efficacy vaccine. I say this because 94 events is a lot of data for a vaccine trial, and even more so when efficacy exceeds 90%.
- Pfizer's first analysis was planned for 32 events, which they pushed back after discussions with FDA. But by the time they analyzed the data, 94 had accrued. This shows how quickly trials can generate results when placed in hotspots (and how much transmission is ongoing!)
- While the results are exciting, of course we will want to independently evaluate them. Unlike treatments, promising data from vaccines do not immediately change standard of care. The vaccines will undergo a rigorous review process first which will play out over time.
- Things I will be most interested in seeing:
- How well does the vaccine prevent severe disease?
- How well does the vaccine prevent infection?
- How well does the vaccine work across different subgroups (e.g. the elderly)?
We also do not want to interfere with the conduct of other ongoing trials. Where Pfizer's product remains limited, placebo-controlled follow-up is critical. We will need many vaccines to meet demand, including vaccines that can be delivered to resource-limited settings.
-But overall this is great news for Pfizer and for other vaccines in development, as they are targeting the same protein. And it is a testament to just how quickly this research is moving.
https://science.sciencemag.org/content/370/6518/763
Last edited by Amber G. on 14 Nov 2020 01:48, edited 2 times in total.
Re: Wuhan Coronavirus Resource Thread
Those who are really interested should really check out good resources for Vaccine efficacy 101 (or basic bio-statistics) to get understanding of
- How is vaccine efficacy calculated?
- Distinguishing between infection, disease, & severe disease.
- Measuring reduced infectiousness.
- Vaccine efficacy vs. effectiveness.
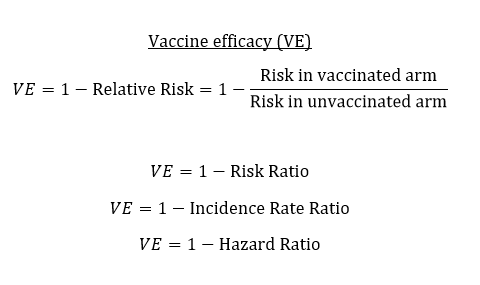
People sometimes talk about vaccine efficacy as a single number, there are actually several different types of vaccine efficacy, such as:
- Efficacy to prevent infection (sterilizing immunity)
- Efficacy to prevent disease
- Efficacy to prevent severe disease
... they also loosely interchange efficacy with effectiveness.
- How is vaccine efficacy calculated?
- Distinguishing between infection, disease, & severe disease.
- Measuring reduced infectiousness.
- Vaccine efficacy vs. effectiveness.

People sometimes talk about vaccine efficacy as a single number, there are actually several different types of vaccine efficacy, such as:
- Efficacy to prevent infection (sterilizing immunity)
- Efficacy to prevent disease
- Efficacy to prevent severe disease
... they also loosely interchange efficacy with effectiveness.
Re: Wuhan Coronavirus Resource Thread
https://www.indiatvnews.com/news/india/ ... -19-664726
India will get 100 million Oxford vaccine shots by December: Adar Poonawalla
Serum Institute of India, the world's largest vaccine maker, is ramping up production of AstraZeneca's Covid-19 shot to have 100 million doses ready by December for an inoculation drive expected to begin across India that same month.
India will get 100 million Oxford vaccine shots by December: Adar Poonawalla
Serum Institute of India, the world's largest vaccine maker, is ramping up production of AstraZeneca's Covid-19 shot to have 100 million doses ready by December for an inoculation drive expected to begin across India that same month.
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
^^^The calculation assumes proper storage and distribution. Hospitals and most government health depts. will do this right. If you're getting the injection at your local pharmacy or WalMart, then it may be an entirely different circumstance.
Re: Wuhan Coronavirus Resource Thread
The Oxford vaccine I think only requires -8C for storage, should be easily done by local pharmacies. The Pfizer one requires something closer to -80C, which is much tougher.
Re: Wuhan Coronavirus Resource Thread
folks with knowledge of pharma trials, is there a 'placebo' arm in human trials of vaccines ?
IOW, are there people in the study who do not recive the vaccine candidate but are exposed to COVID ?
IOW, are there people in the study who do not recive the vaccine candidate but are exposed to COVID ?
Re: Wuhan Coronavirus Resource Thread
Yes, except generally no one can be deliberately 'exposed' to COVID. That would be unethical (because of emergency there was one trial in UK where people were deliberately exposed). That is the reason Pfizer had to wait until some particular no got sick with the virus before they could analyze the data. When they did reach that number (43?) they did not know if some of them got the placebo or the vaccine as it is supposed to be double blind trial i.e. neither the person receiving nor the person administering the vaccine would know if it is placebo or the real one until the data was analyzed. It took time before they could release the results of its efficacy.Rahul M wrote:folks with knowledge of pharma trials, is there a 'placebo' arm in human trials of vaccines ?
IOW, are there people in the study who do not recive the vaccine candidate but are exposed to COVID ?
Re: Wuhan Coronavirus Resource Thread
Bharat Biotech’s COVID-19 vaccine “Covaxin” enters phase-3 trials
Covid-19 vaccine, Covaxin, being developed by Bharat Biotech is now undergoing phase-3 trials, Krishna Ella, Chairman and Managing Director, Bharat Biotech said on Monday. Speaking virtually at a programme organised by the Indian School of Business, Ella said the company is also working on another vaccine for COVID-19 which would be in the form of nasal drops and can be ready by next year.
“We partnered with ICMR for COVID-19 vaccine as we speak it entered the phase 3 trials,” he said. Bharat Biotech is the only vaccine company in the world which has BSL3 production facility (Biosafety level 3), he said. Last month the vaccine maker said it had successfully completed interim analysis of Phase I and II trials of the vaccine and is initiating Phase-III trials in 26,000 participants.
Covaxinis being developed byBharatBiotech, in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). The city-based vaccine maker had on October 2 sought the Drug Controller General of India’s (DCGI) permission to conduct phase 3 randomised double-blind placebo-controlled multicentre trial of its COVID-19 vaccine, sources said.
“We are working on another vaccine through nasal drops my feeling is by next year it will reach the population,” Ella said. BharatBiotechin September said it entered into a licensing agreement with Washington University School of Medicine in St. Louis for a novel “chimp-adenovirus” (Chimpanzee adenovirus), single dose intranasal vaccine for COVID-19.
Re: Wuhan Coronavirus Resource Thread
Allow me to add to previous posts - a good explanation for those with mathematical background about statistics of these trials.Rahul M wrote:folks with knowledge of pharma trials, is there a 'placebo' arm in human trials of vaccines ?
IOW, are there people in the study who do not recive the vaccine candidate but are exposed to COVID ?
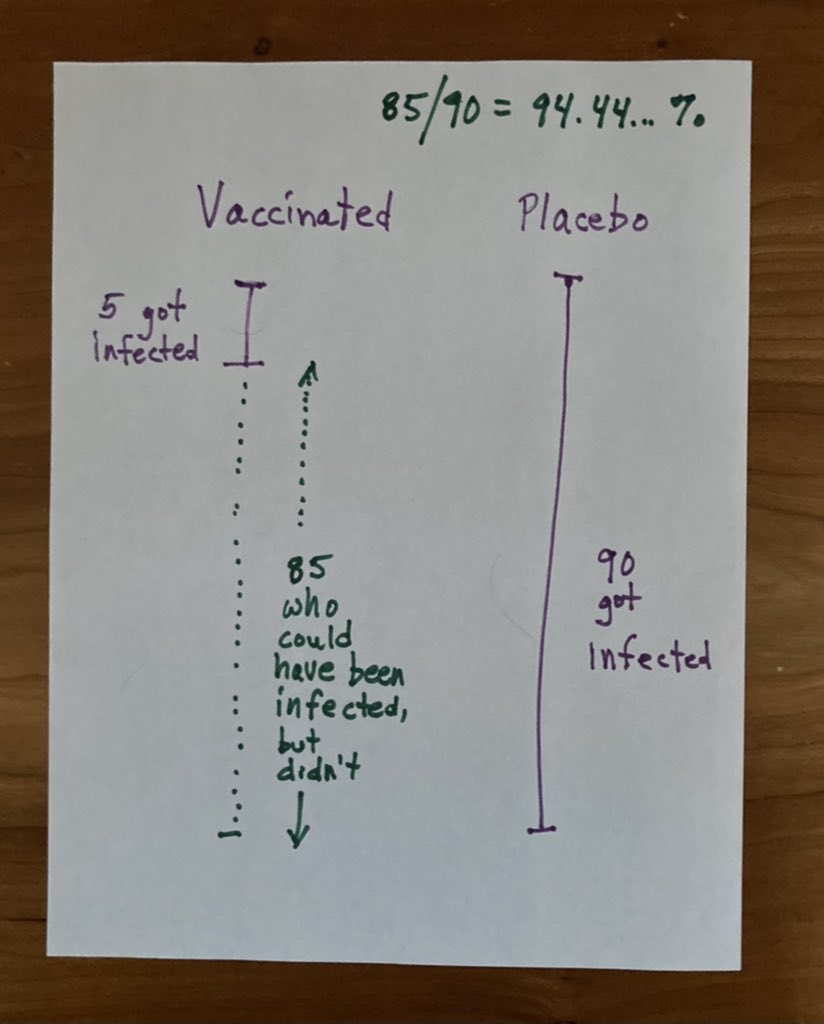
Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study
(For perspective, the study enrolled more than 30,000 people)DSMB reported that the candidate was safe and well-tolerated and noted a vaccine efficacy rate of 94.5%. The findings are statistically significant, meaning they are likely not due to chance. 90 of the cases occurred in the placebo group and 5 occurred in the vaccinated group. There were 11 cases of severe COVID-19 out of the 95 total, all of which occurred in the placebo group.
(Another good reference is NIH site: : https://www.nih.gov/news-events/news-re ... 19-vaccine)
The number which *many* are reporting 94.5% comes from 85/90 (which actually rounds up to 94.4% (
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
So the placebo group had 90/90 = 100% got Covid.
The vaccinated group had 5/90 = 5.6% got Covid.
So what is the definition of effective rate and efficacy rate by CDC definition?
The vaccinated group had 5/90 = 5.6% got Covid.
So what is the definition of effective rate and efficacy rate by CDC definition?
Re: Wuhan Coronavirus Resource Thread
NO..please see my earlier post(s) - (references - Dean's writeup is particularly good - any good text book or review article is good).Mort Walker wrote:So the placebo group had 90/90 = 100% got Covid.
The vaccinated group had 5/90 = 5.6% got Covid.
So what is the definition of effective rate and efficacy rate by CDC definition?
Basically what is says (in very simple terms) after tens of thousands of people involved, statistically speaking in a group of 90 people who got covid, 85 would would have not gotten it if they had vaccine - and all other things were equal.
(Of course, what is even more important is, they have not found - in these trials - any negative effect so they can think that vaccine is safe - confidence in safety is very high).
Last edited by Amber G. on 16 Nov 2020 23:39, edited 1 time in total.
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
I just read the CDC report. It doesn’t state total sample size in the thousands.
Re: Wuhan Coronavirus Resource Thread
Just check the NIH article I posted above, for example:Mort Walker wrote:I just read the CDC report. It doesn’t state total sample size in the thousands.
More than 30,000 participants at 100 clinical research sites in the United States are participating in the study, which launched on July 27, 2020, after results from earlier stage clinical testing indicated that the vaccine candidate is well-tolerated and immunogenic....
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
^^^Yes, I understand there are 30,000 participants, but are all of these participants for the Moderna vaccine or participants for all the vaccines including Pfizer?
Also - when you mention confidence in safety are implying a confidence interval?
I wish the NIH would lay this out in a table as opposed to lots of verbiage.
Also - when you mention confidence in safety are implying a confidence interval?
I wish the NIH would lay this out in a table as opposed to lots of verbiage.
Re: Wuhan Coronavirus Resource Thread
Pfizer trials - per them - (again see my earlier Pfizer's report) are about 44,000 total participants enrolled in that trial - details about how many got placebo or vaccine - what was their age/group race etc consists of lot of details and that's why these kind of studies require good mathematical modeling etc to calculate (or estimate with high confidence) the effectiveness and safety.,,The trials involve lot of blind (or double blind) practices where even Pfizer do not know who got placebo or vaccine etc.Mort Walker wrote:^^^Yes, I understand there are 30,000 participants, but are all of these participants for the Moderna vaccine or participants for all the vaccines including Pfizer?
Pfizer (and many other Indian and US companies), by the way, has been sharing this data quite openly. One can also enquire detail clinical data and they have been good to provide it when asked. ClinicalTrials.gov in US Clinical Trials Register in EU has current detail data and NIH and CDC etc have their own summary/analysis etc when the data is available. (Some of the Trump's recent admin has been quite bad in sharing the analysis of data but hope that will change soon)
Last edited by Amber G. on 17 Nov 2020 00:35, edited 2 times in total.
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
Ok. Thank you. It is very helpful trying to understand this.
I still don’t understand the differences between effective rate vs. efficacy rate. Could you briefly explain in this context?
I still don’t understand the differences between effective rate vs. efficacy rate. Could you briefly explain in this context?
Re: Wuhan Coronavirus Resource Thread
As said before there are several different types of vaccine efficacy, people talk about such as:Mort Walker wrote:Ok. Thank you. It is very helpful trying to understand this.
I still don’t understand the differences between effective rate vs. efficacy rate. Could you briefly explain in this context?
- Efficacy to prevent infection (sterilizing immunity)
- Efficacy to prevent disease
- Efficacy to prevent severe disease.
But in all cases it = 1 - relative relative risk.
In short simple terms the difference between efficacy and effective rate is:
efficacy - in controlled trials the result you get.
effective rate - in real world what would one expect. (external factors are taken into account).
Here the real world means - how good your distribution system is .. how many people will take vaccines properly ..etc..
For, example, for Pfizer , one aspect that the vaccines have to be kept at very low temperature can have quite a bit effect on effective rate. (what if some doses were not kept at that low temperature?).
Here is a primer from NIH:
Last edited by Amber G. on 17 Nov 2020 01:22, edited 1 time in total.
Re: Wuhan Coronavirus Resource Thread
https://edition.cnn.com/2020/11/10/euro ... index.html
Turkish German couple credited for Pfizer vaccine
Turkish German couple credited for Pfizer vaccine