No France? They were supposed to be the darling of India in Europe and we were supposed to outsource our defense industry to them no?Cyrano wrote:Thank you Arshyam garu.
Meanwhile, Covid-19: 8 European countries include Covishield in 'green pass'
Wuhan Coronavirus Resource Thread
Re: Wuhan Coronavirus Resource Thread
Re: Wuhan Coronavirus Resource Thread
France will do the same, just processing time of a few days.
Re: Wuhan Coronavirus Resource Thread
Uttam wrote:Zydus covid vaccine shows 66.6% efficacy, applies for EUA
In the interim analysis, the pharmaceutical company said, the primary efficacy of the vaccine is 66.6% for symptomatic RT-PCR positive cases. The company further said that the vaccine is also “safe and very well tolerated" in the adolescent population in the 12-18 years age group.
No moderate case of covid-19 was observed in the vaccine arm post administration of the third dose suggesting 100% efficacy for moderate disease. No severe cases or deaths due to covid-19 occurred in the vaccine arm after administration the second dose of the vaccine," the company said in a statement.
Some more details here.
Can Launch Vaccine in 45-60 Days Once Approval Comes, Says Zydus Cadila on World's 1st Plasmid DNA Shot
Re: Wuhan Coronavirus Resource Thread
It is either all 27 countries or nothing. So jut wait. Usual suspect Germany is the main obstacle. If they have their way they would throw out European developing countries out of EU. UK will follow the line.Cyrano wrote:Thank you Arshyam garu.
Meanwhile, Covid-19: 8 European countries include Covishield in 'green pass'
NEW DELHI: Amid the ongoing tussle between India and the European Union over the “green pass” for Covid vaccines, eight European countries have put Covishield on their list of approved vaccines, the Economic Times reported on Thursday.
Eight European countries — Germany, Slovenia, Austria, Greece, Iceland, Ireland, Spain and Switzerland — have included Covishield on their list of approved vaccines. This means those inoculated with the Covishield vaccine will be exempted from travel curbs to these countries.
On Wednesday, India had said it would recognise EU’s digital Covid certificate on a reciprocal basis and would not accept it until the EU does the same for Indian vaccines — Covishield and Covaxin.
India, which had raised the issue with the European Medicines Agency (EMA) and France, had also asked EU member states to individually consider extending a similar exemption to those who have had Covishield and Covaxin jabs.
The 'green pass', which will be required for travel in the EU from July 1, will exempt those who have taken two doses of a vaccine from mandatory quarantine.
Issue blown out of proportion: Adar
Serum Institute of India CEO Adar Poonawalla played down on Wednesday with Covishield not getting approved for the EU green pass, saying, “It is not a controversy at all. It’s been blown out of proportion,” reports Naomi Canton.
“The European Medicines Agency (EMA) is correct in asking us to apply, which we have through AstraZeneca a month ago, and that process has to take its time. In a month we are confident the EMA will approve Covishield. There is no reason not to as it is based on AstraZeneca data and our product is identical to AstraZeneca more or less,” Poonawalla said.
Re: Wuhan Coronavirus Resource Thread
These are hard systems to build/design. There will always be talk about digital divide whenever there is a human to machine interaction. I would like to think workflow design was under the assumption that there would be sufficient vaccines available for everyone. Long term that may very well be true so the system could work very well moving forward. I feel It was never designed considering the inventory available and delivery mechanism when supply is smaller than demand. This is no way to be taken as failure, but another use case to be considered while designing user experience for managing delivery efficiently.disha wrote:
pgbhat'ji, lot of this developments are learn as you go. We desis are extremely thin on patience, easily go into a rona-dhona mode and matrix comparison and quick to jump and point out lacunae to get unnecessary attention.
CoWin is a winner when 50+ countries want to adopt it. Some 1st world nations do not have any vaccines leave alone an app to manage the vaccinations and some 2nd world country like US and fUK have worse issues than people point out with their CoWin app.
For example, when I got vaccinated, the portal could not even recognize the nearest pharmacy by pin (zip) code. And ppl had to travel miles to get vaccination. Now I regret it. If I had shown vaccine hesitancy, then I might have had a shot at several hundered thousand dollar prize money.
Re: Wuhan Coronavirus Resource Thread
Day 167: https://www.pib.gov.in/PressReleseDetai ... ID=1732026
Day 166: https://www.pib.gov.in/PressReleseDetai ... ID=1732026
33,96,28,356 - 33,54,69,340 = 41,59,016
The increased supple starting July that was reported in PIB late June appears to be on track.
Day 166: https://www.pib.gov.in/PressReleseDetai ... ID=1732026
33,96,28,356 - 33,54,69,340 = 41,59,016
The increased supple starting July that was reported in PIB late June appears to be on track.
-
Mort Walker
- BRF Oldie
- Posts: 10040
- Joined: 31 May 2004 11:31
- Location: The rings around Uranus.
Re: Wuhan Coronavirus Resource Thread
^^^If BB can get the nasal spray out, then it will be a game changer for numbers vaccinated.
Re: Wuhan Coronavirus Resource Thread
I heard in Hyd that Govt. hospitals ran out of covishieild until July 3.
Re: Wuhan Coronavirus Resource Thread
12 crore Covid vaccine doses to be provided to states, UTs this month
Source:
Source:
Union Health Minister Harsh Vardhan
India has planned to provide 12 crore doses of Covid-19 vaccines this month in government as well as private hospitals across 37 states and Union Territories (UTS) covering people above 18 years of age.
This means the total supply for July will be about 16 crores, averaging about 5.1 million/day in July."Total of 12 crore doses shall be made available in July. Private hospital supply will be over and above this," Vardhan said in a tweet.
Looks like there is still time before Covaxin supply sees a major ramp-up.10 crore doses of Covishield and two crore doses of Covaxin will be allocated to the states and the UTs this month
Re: Wuhan Coronavirus Resource Thread
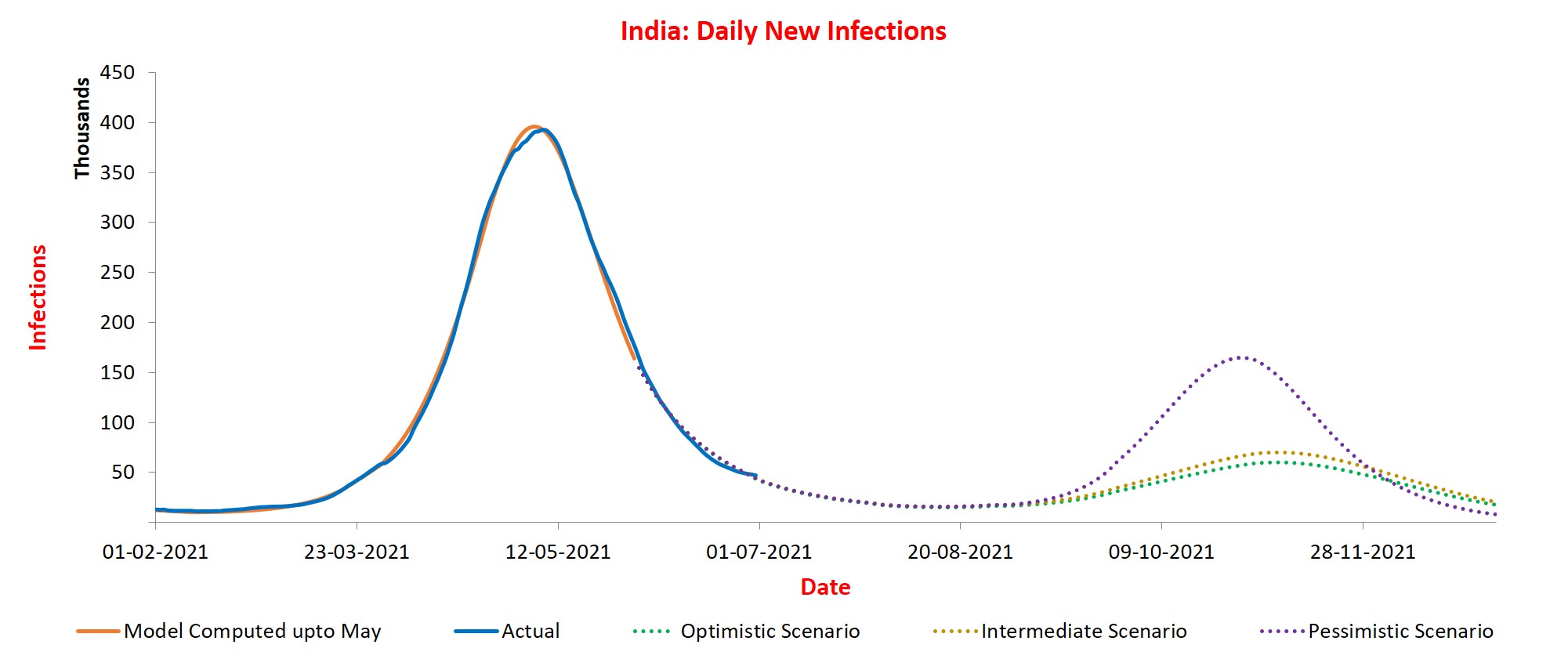
From SUTRA Model:
(Credit: Prof Agrawal, Vidyasagar et-al)
Three scenarios:
(Blue is actual data. Orange one is model prediction until May. Dotted curves are three scenarios plotted from June onwards)
S1 - Optimistic - life goes back to normal by August, and there is no new mutant.
S2 - Intermediate - Vaccination is 20% less effective in addition to optimistic scenario assumptions.
S3 - Pessimistic - A new, 25% more infectious variant spreads in August. (Delta + is NOT more infectious then Delta)
We are assuming the vaccine drives goes on (in what we think will happen - 5 M/day average now soon to be going to 10M / day etc). Also loss of immunity is what our data/medical research has shown.
(Credit: Prof Agrawal, Vidyasagar et-al)
Three scenarios:
(Blue is actual data. Orange one is model prediction until May. Dotted curves are three scenarios plotted from June onwards)
S1 - Optimistic - life goes back to normal by August, and there is no new mutant.
S2 - Intermediate - Vaccination is 20% less effective in addition to optimistic scenario assumptions.
S3 - Pessimistic - A new, 25% more infectious variant spreads in August. (Delta + is NOT more infectious then Delta)
We are assuming the vaccine drives goes on (in what we think will happen - 5 M/day average now soon to be going to 10M / day etc). Also loss of immunity is what our data/medical research has shown.

Re: Wuhan Coronavirus Resource Thread
Day 168: https://www.pib.gov.in/PressReleseDetai ... ID=1732372
Day 167: https://www.pib.gov.in/PressReleseDetai ... ID=1732026
34,41,00,158 - 33,96,28,356 = 44,71,802
Back to over 4m for the first couple of days of the month.
Day 167: https://www.pib.gov.in/PressReleseDetai ... ID=1732026
34,41,00,158 - 33,96,28,356 = 44,71,802
Back to over 4m for the first couple of days of the month.
Re: Wuhan Coronavirus Resource Thread
^^^ >>> There is not much difference between S1 (optimistic) and S2 (intermediate) ==> vaccine efficacy changes do not have significant impact ==> Recommendation to GoI and powers to be, go FULL speed with vaccinations...The peak could be even lower if we increase the vaccination speed.
For aam janata - Masks, Ventilation, Vaccinations, and avoiding the crowd is still the BEST way to protect you and your family.
- A faster spreading mutant has bigger impact (but even this is nowhere close to second wave)
Bottom line: If there is no significantly faster spreading mutant, third wave will be a ripple. And if there is such a mutant, third wave will be comparable to first one.
However, if there is an immunity-escape mutant, all the above scenarios will be invalid!
So people - PLEASE make sure that we do masking and vaccines etc and prevent spread/mutation.
For aam janata - Masks, Ventilation, Vaccinations, and avoiding the crowd is still the BEST way to protect you and your family.
- A faster spreading mutant has bigger impact (but even this is nowhere close to second wave)
Bottom line: If there is no significantly faster spreading mutant, third wave will be a ripple. And if there is such a mutant, third wave will be comparable to first one.
However, if there is an immunity-escape mutant, all the above scenarios will be invalid!
So people - PLEASE make sure that we do masking and vaccines etc and prevent spread/mutation.
Re: Wuhan Coronavirus Resource Thread
Rant
Whenever I see these graphs I get confused as I can not properly read them. Being color blind it takes a lot of effort to make out which line is which. I wish graph designers also use symbols on top of the lines, like circles, squares etc. Considering that almost 9% are colorblind (8% men and 1% women) we do form a large group.
I have changed my computer setting to color blindness friendly and even spent $400 to get color correcting glasses. These do help me in passing Ishiharas plate test but not that much of help as they only SHIFT the colors 1.e. if you are blind to two colors without glasses, you will be blind two different colors with the glasses.
End of rant.
Whenever I see these graphs I get confused as I can not properly read them. Being color blind it takes a lot of effort to make out which line is which. I wish graph designers also use symbols on top of the lines, like circles, squares etc. Considering that almost 9% are colorblind (8% men and 1% women) we do form a large group.
I have changed my computer setting to color blindness friendly and even spent $400 to get color correcting glasses. These do help me in passing Ishiharas plate test but not that much of help as they only SHIFT the colors 1.e. if you are blind to two colors without glasses, you will be blind two different colors with the glasses.
End of rant.
Re: Wuhan Coronavirus Resource Thread
Prof. Agrawal is accessible on twitter - you can write feedback on this to him. The www.sutra-india.in site is developed by an IITK incubated entity so he can probably pass on the information.
Re: Wuhan Coronavirus Resource Thread
Today U.S. announced that it will be shipping about 40 million doses by the end of this week.
The countries include:
Korea, Mexico, Canada, Brazil, Taiwan, Honduras, Colombia, Pakistan, Peru, Ecuador, Malaysia, & Bangladesh.
(J&J to Brazil and Columbia, Pfizer to Peru ,Ecuador and Malaysia, and Moderna to Pak, Bangladesh and the rest).
The countries include:
Korea, Mexico, Canada, Brazil, Taiwan, Honduras, Colombia, Pakistan, Peru, Ecuador, Malaysia, & Bangladesh.
(J&J to Brazil and Columbia, Pfizer to Peru ,Ecuador and Malaysia, and Moderna to Pak, Bangladesh and the rest).
Re: Wuhan Coronavirus Resource Thread
FWIE: Some of my close family members are visually impaired/blind so am familiar with issues. Good hardware/software enabled them to solve such problems (some have successfully went on to work in scientific field). Few points may be of help:saip wrote:Rant
Whenever I see these graphs I get confused as I can not properly read them. Being color blind it takes a lot of effort to make out which line is which. I wish graph designers also use symbols on top of the lines.. <snip>
- Many places (eg Facebook, Twitter etc) allow 'alt-text' and thus some/many people like me who put graphs (of picture) use this feature to put description ..... some help to look at the graph if you have the right software.
- Sutra-India, for example has an option, where you can load the data (of those graphs) in Excel spread-sheet.
- There are AI software (eg Microsoft's "seeing AI") which are becoming more and more intelligent to describe pictures and graphs in general.
---
Coming back to SUTRA graph I posted (see IITK's site for more detail graphs) the important part is:
Bottom line: If there is no significantly faster spreading mutant, third wave will be a ripple. And if there is such a mutant, third wave will be comparable to first one. (Timing is late October) -- There is not much difference between S1 (optimistic) and S2 (intermediate).
However, if there is an immunity-escape mutant, all the above scenarios will be invalid! .. graph does not matter
Re: Wuhan Coronavirus Resource Thread
Suraj and Amber G, thanks. Windows does have color filters to help color blind. Correcting glasses help too. The only advantage is many times we see through camouflage. In fact the US used color blind people in Vietnam. My colleague was one of them.
Re: Wuhan Coronavirus Resource Thread
Meanwhile some taza data on B&B's Cowaxin:
Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a double-blind, randomised, controlled phase 3 trial
>>12: Efficacy: Overall 77.8% (95% CI: 65.2,86.4)
(lower from the 81% reported earlier, but not bad)
After 2 doses,
A. Symptomatic cases= 93.4%
B. Asymptomatic cases = 63.6%
C: Severe cases: 93.4%
D: Against the Delta variant: 65.2%
E: Total efficacy in 60+ yo: 67·8% for 1858 total in the group
F: Total efficacy in young under 60 yo: 79·4% for 15,115 total in the group
-----Side effects:
A: No "statistically significant" differences in serious adverse events between vaccine & placebo
Total serious events in 99- 39 in vaccine & 60 in placebo.
B: No cases of anaphylaxis or vaccine-related deaths
C: 2 serious adverse events reported
D: Most reported side effects:
headache, pyrexia, fatigue and myalgia in less than 1% in both groups.
E: Rates of local and systemic side effects reported (vaccine vs placebo):
mild 11·2% vs 10·8%
moderate 0·8% vs 1·1%
severe 0·3% vs 0·4%
Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a double-blind, randomised, controlled phase 3 trial
>>12: Efficacy: Overall 77.8% (95% CI: 65.2,86.4)
(lower from the 81% reported earlier, but not bad)
After 2 doses,
A. Symptomatic cases= 93.4%
B. Asymptomatic cases = 63.6%
C: Severe cases: 93.4%
D: Against the Delta variant: 65.2%
E: Total efficacy in 60+ yo: 67·8% for 1858 total in the group
F: Total efficacy in young under 60 yo: 79·4% for 15,115 total in the group
-----Side effects:
A: No "statistically significant" differences in serious adverse events between vaccine & placebo
Total serious events in 99- 39 in vaccine & 60 in placebo.
B: No cases of anaphylaxis or vaccine-related deaths
C: 2 serious adverse events reported
D: Most reported side effects:
headache, pyrexia, fatigue and myalgia in less than 1% in both groups.
E: Rates of local and systemic side effects reported (vaccine vs placebo):
mild 11·2% vs 10·8%
moderate 0·8% vs 1·1%
severe 0·3% vs 0·4%
Re: Wuhan Coronavirus Resource Thread
Finally the Ph 3 trials are published for Covaxin, good to see this.
Re: Wuhan Coronavirus Resource Thread
Bharat Biotech Concludes Final Analysis for COVAXIN® Efficacy from Phase 3 Clinical Trials
● Efficacy analysis demonstrates COVAXIN® to be 77.8% effective against symptomatic COVID-19, through evaluation of 130 confirmed cases, with 24 observed in the vaccine group versus 106 in the placebo group
● Efficacy analysis demonstrates COVAXIN® to be 93.4% effective against severe symptomatic COVID-19
● Safety analysis demonstrates adverse events reported were similar to placebo, with 12% of subjects experiencing commonly known side effects and less than 0.5% of subjects feeling serious adverse events
● Efficacy data demonstrates 63.6% protection against asymptomatic COVID-19
● Efficacy data demonstrates 65.2% protection against the SARS-CoV-2, B.1.617.2 Delta variant
Hyderabad, July 3, 2021: Bharat Biotech, a global leader in vaccine development and innovation, announced today safety and efficacy analysis data from Phase III clinical trials of COVAXIN®, a whole virion inactivated vaccine against SARS-CoV2, was developed in partnership with ICMR and NIV Pune.
Phase 3 clinical trials of COVAXIN® was an event driven analysis of 130 symptomatic COVID-19 cases, reported at least two weeks after the 2nd dose, conducted at 25 sites across India. COVAXIN® is formulated with a novel Algel+IMDG adjuvant. IMDG is a TLR7/8 agonist known to induce memory T cell responses along with strong neutralizing antibodies. The activation of cell mediated immune responses is especially valuable in a multi epitope vaccine such as COVAXIN®, where immune protection can be achieved from S, RBD and N proteins alike. IMDG was developed under partnership between Virovax and NIAID, National Institutes of Health USA.
COVAXIN® was well tolerated and the Data Safety Monitoring Board has not reported any safety concerns related to the vaccine. The overall rate of adverse events observed in COVAXIN® was lower than that seen in otherCovid-19 vaccines. The safety profile of COVAXIN® is now well established based on inactivated vaccines technology, and in large part due to the extensive 20-year safety track record of Bharat Biotech’s vero cell manufacturing platform. Furthermore, Bharat Biotech has so far not sought indemnity for COVAXIN® from the Governments.
No licensed SARS-CoV-2 vaccine has reported efficacy against asymptomatic infection in a randomised controlled trial, based on qPCR testing. COVAXIN® is the first to report promising efficacy against asymptomatic infections based on qPCR testing that will help in reducing disease transmission.
Dr. Krishna Ella, Chairman & Managing Director, Bharat Biotech, said, “The successful safety and efficacy readouts of COVAXIN® as a result of conducting the largest ever COVID Vaccines trials in India establishes the ability of India and developing world countries to focus towards innovation and novel product development. We are proud to state that Innovation from India will now be available to protect global populations.”
COVAXIN® has been specifically designed to meet the needs of global distribution chains, the requirements for which are more critical in low- and middle-income countries. It has been formulated to enable shipping and long-term storage at 2-8ºC. It is also formulated to adhere to a multi-dose vial policy, thereby reducing open vial wastage, saving money to procurement agencies and governments alike.
Prof. (Dr) Balram Bhargava, Secretary Department of Health Research & Director General Indian Council of Medical Research, said, “I am delighted to note that COVAXIN®, developed by ICMR and BBIL under an effective public private partnership, has demonstrated an overall efficacy of 77.8% in India’s largest COVID phase 3 clinical trial thus far. Our scientists at ICMR and BBIL have worked tirelessly to deliver a truly effective vaccine of highest international standards. COVAXIN® will not only benefit the Indian citizens but would also immensely contribute to protect the global community against the deadly SARS-CoV-2 virus. I am also pleased to see that COVAXIN® works well against all variant strains of SARS-CoV-2. The successful development of COVAXIN® has consolidated the position of Indian academia and Industry in the global arena.”
Bharat Biotech is a company driven by science and validated by empirical evidence. Its commitment to data transparency has been proven again with 10 publications on COVAXIN®, covering all aspects of product development, all within 12 months.
Bharat Biotech’s commitment to continued improvement of COVAXIN® is well under way with additional clinical trials to establish safety and efficacy in children between 2-18 years of age. A clinical trial to determine the safety and immunogenicity of a booster dose is also in process. Several research activities are being carried out to study variants of concern and to assess their suitability for follow up booster doses.
Mrs. Suchitra Ella, Joint Managing Director, Bharat Biotech, said, “It is a momentous day for everyone, at Bharat Biotech, as we announce the Final Phase-3 Results of COVAXIN® and its efficacy of 77.8%. We wish to thank ICMR, NIV-Pune, Virovax, DSMB and Adjudication Committee. We earnestly thank our clinical trial sites, Principle Investigators, IQVIA, and every participant who has reposed their faith in COVAXIN®. We SINCERELY thank all our employees for enduring work pressures through the pandemic & lockdowns, with 24x7 commitment amidst unprecedented number of physical challenges, stress and continuous operations. We specially thank our medical affairs team for leading the project, the technical and marketing teams who have relentlessly worked to complete the clinical trials and coordination of 25 sites across the country since May 2020.”
COVAXIN® has been evaluated through neutralizing antibody responses against several variants of concern, namely B.1.617.2 (Delta), B.1.617.1 (Kappa), B.1.1.7 (Alpha), B.1.351 (Beta), P2- B.1.1.28 (Gamma). The data from these studies have been extensively published in peer reviewed journals and available for review in the public domain.
Prof. (Dr) Priya Abraham, Director National Institute of Virology ICMR said, “The overall efficacy of 77.8 % following the phase III clinical trial of COVAXIN® is wonderful news. ICMR-NIV and BBIL have had very fruitful interactions during this exhilarating journey. Sera from COVAXIN® recipients have also been evaluated against viral variants detected in India i.e., the Alpha, Beta, Zeta, Kappa and Delta. The making of this vaccine entirely on Indian soil is a matter of great pride to every Indian”
COVAXIN® has now received emergency use authorizations in 16 countries including, Brazil, India, Philippines, Iran, Mexico, etc. with EUA’s in process in 50 countries worldwide. The company is in discussions with WHO to obtain emergency Use Listing for COVAXIN®. The product has been exported to several countries with additional requests for supplies being received.
Bharat Biotech has established COVAXIN® manufacturing at 4 facilities within India, further expansions are in process to reach an annualized capacity of 1 billion doses by the end of 2021. Technology transfer activities are in progress to companies in United States, and other countries.
More about COVAXIN® - https://www.bharatbiotech.com/covaxin.html
Read the full Pre-Print Publication here: https://www.medrxiv.org/content/10.1101 ... 21259439v1
Re: Wuhan Coronavirus Resource Thread
India’s COVID-19 Vaccination Coverage exceeds 35 Cr
Day 169: https://pib.gov.in/PressReleasePage.aspx?PRID=1732551
Day 168: https://www.pib.gov.in/PressReleseDetai ... ID=1732372
35,05,42,004 - 34,41,00,158 = 64,41,846
Way to go!
Day 169: https://pib.gov.in/PressReleasePage.aspx?PRID=1732551
Day 168: https://www.pib.gov.in/PressReleseDetai ... ID=1732372
35,05,42,004 - 34,41,00,158 = 64,41,846
Way to go!
Re: Wuhan Coronavirus Resource Thread
The story is in main stream newspapers:
https://www.hindustantimes.com/india-ne ... 22664.html
More vaccines (including mRNA like Moderna etc) are coming into play - Let us all do our part - get vaccinated and tell everyone to listen to science etc.
https://www.hindustantimes.com/india-ne ... 22664.html
To add: In the above calculations - from what I know - the effect due to vaccinations (that is how fast we can distribute vaccines) is taken in a little conservative way (IMO), I think (and hope) the actual speed would be faster. (hoping 70% of adult population by end of September) and this will actually reduce the height of the peak --- we will see, Also hope the we are little more careful than beginning of march and at least 10-20% people keep following covid protocol..)..Amber G. wrote:From SUTRA Model:
(Credit: Prof Agrawal, Vidyasagar et-al)
Three scenarios:
(Blue is actual data. Orange one is model prediction until May. Dotted curves are three scenarios plotted from June onwards)
S1 - Optimistic - life goes back to normal by August, and there is no new mutant.
S2 - Intermediate - Vaccination is 20% less effective in addition to optimistic scenario assumptions.
S3 - Pessimistic - A new, 25% more infectious variant spreads in August. (Delta + is NOT more infectious then Delta)
We are assuming the vaccine drives goes on (in what we think will happen - 5 M/day average now soon to be going to 10M / day etc). Also loss of immunity is what our data/medical research has shown.
More vaccines (including mRNA like Moderna etc) are coming into play - Let us all do our part - get vaccinated and tell everyone to listen to science etc.
Re: Wuhan Coronavirus Resource Thread
Potentially good news, however only about 10-12% of the adult population will have received two doses and be two weeks past the 2nd dose by the time the third wave starts according to the model.
Does this model assume vaccination by 1 dose or by 2 doses?
Does this model assume vaccination by 1 dose or by 2 doses?
Re: Wuhan Coronavirus Resource Thread
^^^ Short answer yes (lots of one doses etc),- I think (in my opinion) it is actually a little conservative, the model, (again I think) is also assuming things like about 12% lose immunity (natural - and not due to vaccines) ) within 4-5 months (data they had for India)..It seems that unless there is a variant with higher transmissivity than delta (Delta+ has the same transmissivity as Delta) the peak would be quite small compared to the horrendous second peak. (If people's behaviour changes to good masking etc (which I am not that optimistic) and vaccinations accelerate as I hope they would, we will be okay.
---
The present version of SUTRA does not have vaccinations built into model yet (work for the next version ) but the effects of vaccinations are nicely taken into account by other parameters like reach (rho).. and model seem to fit with data in countries like UK etc so the basis seems solid.
) but the effects of vaccinations are nicely taken into account by other parameters like reach (rho).. and model seem to fit with data in countries like UK etc so the basis seems solid.
---
The present version of SUTRA does not have vaccinations built into model yet (work for the next version
Re: Wuhan Coronavirus Resource Thread
The chosen ones are already few steps ahead, holding the world hostage again with new stories. Economies around world are under stress from lockdowns and many governments have been changed due to economic malaise (Peru gov changed from rw to socialist, and seeing protests Brazil, Ball-see-naro is next). India must stay on guard, onlee ullah knows what is coming India's way before 2024  majority vaccination should help minimise the damage.
majority vaccination should help minimise the damage.
Question for experts, why do these variants end up mutating primarily in developing countries, taking them on the verge of economic destruction? How does it mutate in developing or underdeveloped country (say Delta), but never further mutate when it ends up in a developed country? <sarcasm>This virus is very racial and kommunal, but very intelligent </sarcasm>
Here is a new kid variant not the block. Lamda variant has been around for 5-6 months, MSM has decided to bubble it up.
Lambda Covid variant’s ‘unusual’ mutations puzzle scientists
Question for experts, why do these variants end up mutating primarily in developing countries, taking them on the verge of economic destruction? How does it mutate in developing or underdeveloped country (say Delta), but never further mutate when it ends up in a developed country? <sarcasm>This virus is very racial and kommunal, but very intelligent </sarcasm>
Here is a new kid variant not the block. Lamda variant has been around for 5-6 months, MSM has decided to bubble it up.
Lambda Covid variant’s ‘unusual’ mutations puzzle scientists
A new coronavirus variant that has infected thousands in South America has now been discovered in the UK.
Classified as a "variant of interest" by the World Health Organization (WHO) on June 17, the lambda variant has been detected in 29 nations — seven of them in Latin America. In Peru, where it was first identified, the lambda variant now accounts for 82% of new infections.
Now, 6 cases of this COVID-19 variant have been found in the UK, all linked to overseas travel.
-
sanjaykumar
- BRF Oldie
- Posts: 6116
- Joined: 16 Oct 2005 05:51
Re: Wuhan Coronavirus Resource Thread
The mutation rate is proportional to cycles of replication
For example if a drug is given that clears the virus eg monoclonal antibody, there will be fewer mutations.
Related to R to population, to duration if infection. And certainly resources available for isolation to break the propagation chain.
That last point may be why there are more mutations reported in poorer countries.
Far from sarcasm, that was actually a good question.
For example if a drug is given that clears the virus eg monoclonal antibody, there will be fewer mutations.
Related to R to population, to duration if infection. And certainly resources available for isolation to break the propagation chain.
That last point may be why there are more mutations reported in poorer countries.
Far from sarcasm, that was actually a good question.
Re: Wuhan Coronavirus Resource Thread
Plus there was a Kent variant as well which was first reported in England.
The more the hosts, the greater the chance of mutation.
The more the hosts, the greater the chance of mutation.
Re: Wuhan Coronavirus Resource Thread
From what I have gathered, the world will not be fully safe from future virus mutations until the new case numbers decrease to manageable/low levels worldwide.
The more the virus is prevalent in the general population the greater the chance of newer mutations. However not all mutations are more dangerous but only the more deadlier ones appear to prevail across the world. Safest thing is for entire world to be fully vaccinated. Since this will take time it is better to be prepared for any eventuality.
Vaccinate our population first then help other countries. The world will need a concerted effort to beat this back and make it more like the seasonal Flu. Which means that in addition to vaccines, we will also need new drugs/medicines that more or less effective even if you catch the virus.
The more the virus is prevalent in the general population the greater the chance of newer mutations. However not all mutations are more dangerous but only the more deadlier ones appear to prevail across the world. Safest thing is for entire world to be fully vaccinated. Since this will take time it is better to be prepared for any eventuality.
Vaccinate our population first then help other countries. The world will need a concerted effort to beat this back and make it more like the seasonal Flu. Which means that in addition to vaccines, we will also need new drugs/medicines that more or less effective even if you catch the virus.
Re: Wuhan Coronavirus Resource Thread
Israel: Pfizer vaccine is only 64% effective against Delta strain after two doses. i.e. - Covaxin is better (64% vs 65.2%)
Ministry data said to show Pfizer shot blocks majority of serious Delta cases
By AMY SPIRO
Today, 10:23 am
The Pfizer-BioNTech COVID vaccine appears to largely prevent hospitalization and serious cases, but is significantly less effective against preventing the spread of the Delta variant of the coronavirus.
New Health Ministry figures reported by the Ynet news site indicated that over the past month, the vaccine, which has been the one used for almost all vaccinated Israelis, has been just 64 percent effective in preventing coronavirus infection. The data reportedly shows that during May, when the strain was less prevalent, the vaccine was 94.3% effective.
According to Ynet, the figures were presented Sunday evening at a meeting of a team of experts advising the government on its handling of the pandemic.
Meanwhile, a study from researchers at the Hebrew University and Hadassah University Medical Center indicated that the Pfizer vaccine is 60-80% effective against infection from the Delta strain.
The Delta variant, which is believed to be twice as contagious as the original strain of COVID-19, is thought to be responsible for 90% of new cases in Israel over the past two weeks. [..]
Re: Wuhan Coronavirus Resource Thread
Day 169: https://pib.gov.in/PressReleasePage.aspx?PRID=1732551 = 35.05 crores
on the morning of Monday 5 July at 7am https://pib.gov.in/PressReleasePage.aspx?PRID=1732724 = 35.28 crores
Therefore doses on Sunday 4 July = 35.28cr - 35.05cr = 23 lakhs
Day 171: https://pib.gov.in/PressReleasePage.aspx?PRID=1732933 = 35.71 cr
Therefore Monday July 5 = 35.71cr - 35.28cr = 43 lakh doses
on the morning of Monday 5 July at 7am https://pib.gov.in/PressReleasePage.aspx?PRID=1732724 = 35.28 crores
Therefore doses on Sunday 4 July = 35.28cr - 35.05cr = 23 lakhs
Day 171: https://pib.gov.in/PressReleasePage.aspx?PRID=1732933 = 35.71 cr
Therefore Monday July 5 = 35.71cr - 35.28cr = 43 lakh doses
Re: Wuhan Coronavirus Resource Thread
https://twitter.com/JaidevJamwal/status ... 1290447873
Scene from Dharmshala, a popular tourist spot for neighbouring state.
Majority of people, most likely tourists are wandering around without a mask and this little boy is asking them to wear one.
It'll not be any loss when these covidiots die. But they'll keep on abusing gobarment for their own mistakes.
Scene from Dharmshala, a popular tourist spot for neighbouring state.
Majority of people, most likely tourists are wandering around without a mask and this little boy is asking them to wear one.
It'll not be any loss when these covidiots die. But they'll keep on abusing gobarment for their own mistakes.
Re: Wuhan Coronavirus Resource Thread
Dr. Raches Ella
@RachesElla
@RachesElla
New data on Delta-Efficacy or Effectiveness (not apple to apple comparison):
Pfizer: Wuhan=95% | Delta=64%
COVAXIN: Wuhan/Delta=78% | Delta=65%
All vaccines prevent >93% of hospitalizations. Regardless of the vaccine, get it, and wear a mask to get to near 100%.
Re: Wuhan Coronavirus Resource Thread
Good of Dr.Ella to put that out there. Pretty brave to take on Big Pharma like that. He feels confident he has Govt backing him.
Re: Wuhan Coronavirus Resource Thread
Thanks for doing this. You have the procedure down perfectly nowKakkaji wrote:Day 169: https://pib.gov.in/PressReleasePage.aspx?PRID=1732551 = 35.05 crores
on the morning of Monday 5 July at 7am https://pib.gov.in/PressReleasePage.aspx?PRID=1732724 = 35.28 crores
Therefore doses on Sunday 4 July = 35.28cr - 35.05cr = 23 lakhs
Day 171: https://pib.gov.in/PressReleasePage.aspx?PRID=1732933 = 35.71 cr
Therefore Monday July 5 = 35.71cr - 35.28cr = 43 lakh doses
Monday Day 171: https://pib.gov.in/PressReleasePage.aspx?PRID=1732724
Tuesday Day 172: https://www.pib.gov.in/PressReleseDetai ... ID=1733195
36,09,56,621 - 35,71,05,461 = 38,51,160
So far this month is doing a few percent better than start of week data from weeks 1-3 of June.
Re: Wuhan Coronavirus Resource Thread
A Few tidbits from SUTRA modeling and reputable news outlets:
Looking at data in India in more details - Delta+ is about the same infectious as Delta and new "phase" is sort of stabilizing. (Many news paper reports about the "third wave" is, IMO, more political guessing than scientific) Unless there is some major new variant pops up (and is vaccine resistant) India should be okay. Increased vaccine rates (and Masking in crowded area etc) could further lower the peak value. Hopefully if we can substantially speed up vaccines (reaching say 600-900 more shots by September which is certainly possible) it would be nice.
In Kerala and Maharashtra things curve is flattening a little slower than those graphs .. but it should stabilize and so will India..
A new variant (Lamda) from Peru is of concern - Data from Peru is being studied but this variant may turn out to be more infectious that Delta. Let us hope it does not spread too fast globally (So far affected more than 30 countries).
Bottom Line: Please get vaccinated and do use masks if you are in crowded area or indoors - *even* as things open up.
(Type of vaccine is not as important as getting one as soon as possible to as many arms as possible)
Looking at data in India in more details - Delta+ is about the same infectious as Delta and new "phase" is sort of stabilizing. (Many news paper reports about the "third wave" is, IMO, more political guessing than scientific) Unless there is some major new variant pops up (and is vaccine resistant) India should be okay. Increased vaccine rates (and Masking in crowded area etc) could further lower the peak value. Hopefully if we can substantially speed up vaccines (reaching say 600-900 more shots by September which is certainly possible) it would be nice.
In Kerala and Maharashtra things curve is flattening a little slower than those graphs .. but it should stabilize and so will India..
A new variant (Lamda) from Peru is of concern - Data from Peru is being studied but this variant may turn out to be more infectious that Delta. Let us hope it does not spread too fast globally (So far affected more than 30 countries).
Bottom Line: Please get vaccinated and do use masks if you are in crowded area or indoors - *even* as things open up.
(Type of vaccine is not as important as getting one as soon as possible to as many arms as possible)
Re: Wuhan Coronavirus Resource Thread
I have a stupid question: viruses mutate all the time and the more the number of hosts, more likely it is to mutate. Additionally, the longer the pool of infected hosts, more the likelihood of mutations. Given the two, how did we manage to have a polio vaccine that is still effective? Same applies to small pox. Polio has been around forever, so did it not mutate as much?
Or is it that we did not do as much gene sequencing of polio virus to identify variants as as are doing for the Wuhan virus?
Or is it that we did not do as much gene sequencing of polio virus to identify variants as as are doing for the Wuhan virus?
Re: Wuhan Coronavirus Resource Thread
Early polio vaccinations were in fact affected by lack of efficacy of the vaccines used - back in the 1970s. There's a great article on the story of India's polio eradication - one that spent decades treading water and finally concluded only during the last decade.
Eradicating poliomyelitis: India's journey from hyperendemic to polio-free status
Eradicating poliomyelitis: India's journey from hyperendemic to polio-free status
The polio eradication effort is portrayed as a success vs Covid vaccination but the reality is quite the reverse - polio immunization was handled poorly for decades.Polio immunization using imported OPV was introduced in Mumbai by the city corporation in 1964 and in Vellore by CMC in 1965. Soon thereafter problems of OPV efficacy were detected and were systematically studied in Vellore31. In 1972, the first definitive study on the problem of low immunogenic efficacy of OPV with standard potency was published32,33. Low vaccine efficacy (VE) was corroborated by counting children developing poliomyelitis in spite of the recommended 3 doses of OPV34. In short, there was ample warning that India had problems with VE of OPV years before, and at the time of the launch of EPI in the country. On the other hand, IPV had showed excellent VE in clinical studies37
India faced a choice. Salk's IPV was widely used from 1955 in USA, Canada, UK and north European countries resulting in rapid control (>95% reduction) of polio. Finland interrupted WPV transmission in 1962 using IPV in campaign mode38. Unfortunately, IPV could not be used to control polio in India as it was not licensed for use even in the private sector. One manufacturer made IPV under Maharashtra State license in 1985/86 but had to discontinue under directions of the Government of India (GOI)31,37. Ultimately IPV was licensed in 2006 when it became apparent that IPV was the vaccine of the future.
According to distributed EPI reports, during 1978-1979, 1979-1980, 1980-1981 and 1981-1982, 27, 24, 24 and 29 million children were injected with DPT, while the numbers of children given three doses of OPV in those years were zero, 0.5 million, 1.3 million and 2.3 million, respectively41.
Re: Wuhan Coronavirus Resource Thread
Short answer for Polio - yes, like others there were mutations/variants and challenges for vaccine makers..( Mainly There are/were three wild types of poliovirus (WPV) – type 1, type 2, and type 3 etc.. and mutation caused problems in eradication etc....any good reference can give more details)..advances in gene-sequencing make identification of variants etc much easier and faster now a days.. which helps to make/improve vaccines faster)Tanaji wrote:I have a stupid question: viruses mutate all the time and the more the number of hosts, more likely it is to mutate. Additionally, the longer the pool of infected hosts, more the likelihood of mutations. Given the two, how did we manage to have a polio vaccine that is still effective? Same applies to small pox. Polio has been around forever, so did it not mutate as much?
Or is it that we did not do as much gene sequencing of polio virus to identify variants as as are doing for the Wuhan virus?
