Meanwhile: ("mild or not")
-
4x kids hospitalizations in NYC (increase in pediatric hospital #COVID19 admissions for Dec 19th versus week of Dec 5th).
-
Hospitalizations in Austin (Texas) are up 138% in one week (My local county (in Ohio) where we are keeping much closer watch situation is quite alarming - lot of active cases and ICU situation already alarming - not surprising 99% is being used by non-vaccinated patients. Somewhat good news is people are becoming more sensible - free testing kits and mask etc, which just a few weeks ago were not much in demand , now attract lines)
-
Pediatric ICU hospitalizations for COVID19 for ages 0-9 also at record high in France.

-
UK hospitals are surpassing even the pessimistic forecasts -- Hospital hospital admissions—all over England (fastest in London area but other places are growing too) now suddenly reporting a about 54% increase in hospitalizations in one week… This is extra concerning because it is now notably **ahead** of LSHTM Omicron hospitalization forecast!
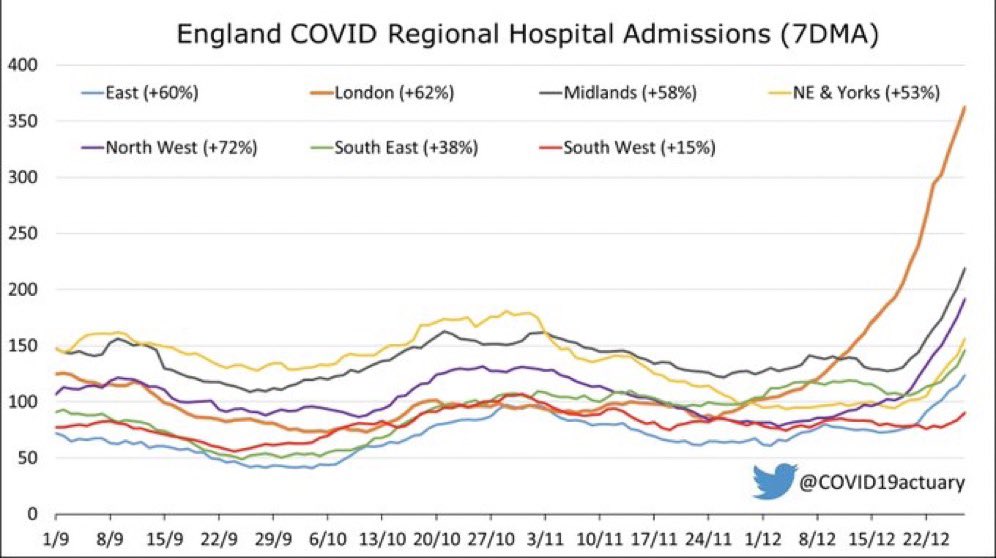
(Keep in mind— the surging in England is there even though they observe much stricter isolation policies than the US’s CDC - Also UK is NOT downplaying it as “mild”?)..
U
S Things are already very concerning ,and likely will see an even worse future trajectory...
Sure there are encouraging scientific reports such as a yet another in vivo study of Omicron infection today, it sure is looking that this variant has less virulence, less chance of inducing Covid pneumonia.
But Hospitals admissions are rising fast..vastly by unvaccinated people. Have heard from *many* who tested +ive even after boosters but serious illness is still predominately among unvaccinated - ---
Here is some data for US:
U.S. COVID update: Surge continues, number in hospital sees biggest jump since July 2020
- New cases: 500 K
- Average: 268 K (+31,754)
- In hospital: 79 K (+5,572)
- In ICU: 17 K (+456)
- New deaths: 2,623
---
-- 7-day average of daily coronavirus cases in the U.S. reaches 267,738, the highest since the pandemic began.
--
Number of Americans hospitalized with COVID-19 tops 76,000, highest since September.
(France to Greece to Australia - every country we are tracking is reporting *highest* increase in one day).
-- Even China - reports 182 new coronavirus cases

, the biggest one-day increase since early 2020, as officials try to contain Xian outbreak. (Note: China is one country - I have to admit where even our best mathematical minds and tools can not sanitize the data or extract useful information)
--- For India the parameters are somewhat stable - In about a month we would see "higher" numbers and peak in later part of February.
Masks are (IMO) very necessary - along with getting as many people vaccinated (boosted etc) in next months as possible.