Re: Wuhan Coronavirus Resource Thread
Posted: 25 May 2020 20:51
Excellent. Let Cheen go ahead and put itself in reverse lockdown mode.Ashokk wrote:China decides to evacuate citizens from India amid rise in coronavirus cases
Consortium of Indian Defence Websites
https://forums.bharat-rakshak.com/
Excellent. Let Cheen go ahead and put itself in reverse lockdown mode.Ashokk wrote:China decides to evacuate citizens from India amid rise in coronavirus cases
So where are the mediapimps now? Why cannot they talk about the spectacular success of testing strategy in India?With supplies of these machines, it would be possible to have Covid-19 testing capacities in districts with no capacity for carrying out the RT-PCR tests, or reverse transcriptase polymerase chain reaction test that is considered the gold standard for detecting Sars-Cov-2 virus.
Am I the only one reading between the lines?Ashokk wrote:China decides to evacuate citizens from India amid the rise in coronavirus cases
You mean Chincoms upping the ante with India? Possible. Sad part is that if they do that, India is on its own. US will make some noises, but nothing beyond that. Hope our military planners and govt are prepared.mappunni wrote:
Am I the only one reading between the lines?
Very unlikely this has anything to do with the border. All sorts of countries are evacuating citizens from India with a lot of flights - but this doesn't make news. You can look on flightradar24 to see incoming passenger flights on international airlines and draw your conclusions. Now China joins this list. It has to be an evacuation flight as long as scheduled flights don't start, and they will in a few weeks but we are talking about China here.mappunni wrote:Am I the only one reading between the lines?Ashokk wrote:China decides to evacuate citizens from India amid the rise in coronavirus cases
You need to look at cases/1M population. With 1.33 billion people and the Chinese as usual hiding their numbers, we will have a lot more cases per day compared to nations with smaller population.CRamS wrote:Coming to the Corona virus itself, its seem India is now the 5th worst affected in terms of # of cases according to TimesNow. This is staggering. And while the recovery rate looks healthy at 42+%, still the rising # of cases is a cause for worry. Not sure what the Indian govt can do. Damned if you do (lock down), damned if you don't. Among all jobs in the world, being the PM of India is the most challenging of them all at this time.mappunni wrote:
Am I the only one reading between the lines?
When has ChiCom provided the real numbers, be it economic or otherwise? It has been very hard to get any facts coming from the ChiComs even in the Pandemic situation. Watching Epoch times, they say there is the second Corona outbreak in two Northern provinces and is much harder to detect until the person ends up gasping for air! Apparently the virus has mutated into 30 different ones now.Ambar wrote:You need to look at the cases/1M population. With 1.33 billion people and the Chinese, as usual, hiding their numbers, we will have a lot more cases per day compared to nations with smaller populations.CRamS wrote:
Coming to the Coronavirus itself, it seems India is now the 5th worst affected in terms of # of cases according to TimesNow. This is staggering. And while the recovery rate looks healthy at 42+%, still the rising # of cases is a cause for worry. Not sure what the Indian govt can do. Damned if you do (lockdown), damned if you don't. Among all jobs in the world, being the PM of India is the most challenging of them all at this time.

sudarshan wrote:US COVID daily deaths as reported day-by-day to worldometer. What the *hic* are they doing to the data, that it's behaving like this? Reporting delays are one possibility, but that regular sawtooth cycle is puzzling.
April 15th was the day the reporting criterion changed. Like I said before, the actual deaths curve had started falling well below the prediction before that date, then the new criterion changed things.
All the predictions are based on epidemiological models and the models do not account for 'local' distribution. For example, the population density of New York is not comparable to the population density of Texas. A county within California cannot be compared to another county. Similarly in India a district cannot be compared to another. On top of it, the movement of people within a bound geographical area is not completely free and completely random.In the early days of the pandemic, limited data availability led the first generation, epidemiological models, to predict that hundreds of millions would be infected with the virus, and millions of lives lost. Fortunately, the reality so far has turned out to be very different.
In essence, identify the superspreaders and isolate them. For example, Thookers and Thooblighis tried to be super spreaders. And they were isolated successfully in some parts of the country and due to political implications, they became hyper spreaders in other parts of the country.research based on the previous Severe Acute Respiratory Syndrome (Sars) epidemic in 2003 has shown that there is a great deal of variability in individual infectiousness. For example, research on Sars epidemic from Singapore revealed that the majority (approximately 73%) of the cases were mildly infectious; in other words, they had an R0 of less than one, while a small proportion of them (approximately 6%) was highly infectious or “super-spreaders” with an R0 > eight.
The concentration of active cases in Ahmedabad is concentrated further into specific wards. It is not Islamophobic to point out that those wards have the highest concentration of Muslims and their cultural practices are contributing both to higher caseloads and higher death rate.Prof Shamika Ravi
@ShamikaRavi
·
17h
Solution to COVID situation in MH cannot be - "But GJ is worse."
1) Case load: 36% (MH) vs. 10% (GJ)
2) New cases: 2500 (MH) vs. 400 (GJ)
3) Death/million: 13.89 (MH) vs. 13.93 (GJ)
4) Cases/100 tests: 13.25% (MH) vs. 7.76%(GJ)
Situation is bad in GJ too, but MH is worse. Focus!
Shivam Singhania
@ShivmSinghania
Replying to
@ShamikaRavi
+ 1) deaths are stabilising in GJ, though still high
2) spread is more concentrated, 80% active cases in Ahmd
3) total and active cases similar to DL with 3x more pop.
4) recovery rate close to 50%, most districts 60-70%, unlike 30% for MH
5) active case load 9% vs 44% MH
Could you look at the date range from April 28th (Tue) to May 10th (Sun)? There's a distinct drop in numbers as the week progresses, then back to a high value the next week. For the observed pattern in that date range, they got to be underreporting a bit more every day, as the week progresses, with max. underreporting on Sat/Sun.williams wrote: Sawtooth cycle is because of underreporting on weekends.
While Sweden might not have an official lockdown, its people were following social distancing and curbing activities that they would normally do. According to reports, its economy is doing no better than others.sudarshan wrote:Sweden finished one round of antibody testing, and found that about 7% of Stockholm had been infected. Lots of press headlines, about how the herd immunity strategy failed, and how long a way it is to the herd immunity threshold of 60%.
The biggest factor affecting inhomogeneity seems to be the velocity of people or people moving about and interacting with each other. By the very definition lock downs and social distancing increases inhomogeneity but free movement of people increases inhomogeneity. So how can we consider HIT during a lockdown and social distancing phase as the real HIT?sudarshan wrote:
The HIT is not set in stone, 60% HIT is based on an extremely simple infection model with an estimated R0 of 2.4. Putting in some realistic assumptions (conservative) about inhomogenities, the HIT comes down to the 10% to 20% range. If the inhomogenity assumptions are not so conservative (more in line with observed CV parameters of around 3.1 to 3.2 for COVID) the HIT comes down below 10% even, with the same R0 of 2.4. In a country with a low population density to begin with, R0 might not even be as high as 2.4.
The point is, public policy should not be driven by a rigid assumption (such as "HIT=60%"), especially when that assumption comes from a naive, first-order mathematical model. Assumptions need to be revised based on fresh data. I do see some acknowledgment of that fact from the scientists, but the press seems stuck on old assumptions. And it's looking like the press is what drives policy.hanumadu wrote:While Sweden might not have an official lockdown, its people were following social distancing and curbing activities that they would normally do. According to reports, its economy is doing no better than others.sudarshan wrote:Sweden finished one round of antibody testing, and found that about 7% of Stockholm had been infected. Lots of press headlines, about how the herd immunity strategy failed, and how long a way it is to the herd immunity threshold of 60%.
Inhomogeneity implies *differences between people for the same parameter*. Social distancing decreases inhomogeneity - everybody stays away from everybody else, everybody does the same thing as everybody else (homogeneous). When people move freely, that's when inhomogeneity matters, because then some people move a lot more than others, etc.hanumadu wrote:The biggest factor affecting inhomogeneity seems to be the velocity of people or people moving about and interacting with each other. By the very definition lock downs and social distancing increases inhomogeneity but free movement of people increases inhomogeneity. So how can we consider HIT during a lockdown and social distancing phase as the real HIT?sudarshan wrote:
The HIT is not set in stone, 60% HIT is based on an extremely simple infection model with an estimated R0 of 2.4. Putting in some realistic assumptions (conservative) about inhomogenities, the HIT comes down to the 10% to 20% range. If the inhomogenity assumptions are not so conservative (more in line with observed CV parameters of around 3.1 to 3.2 for COVID) the HIT comes down below 10% even, with the same R0 of 2.4. In a country with a low population density to begin with, R0 might not even be as high as 2.4.
https://www.covid19india.org/Ambar wrote:Is there a dashboard that tracks daily increase in cases and deaths by state for India similar to what we have for US ? The Ministry of Health dashboard just gives total numbers without day over day comparison. The last 24 hrs have seen the highest daily rise in cases and deaths in India at 7293 and 190 deaths.
Yes, Shamika Ravi's tweets have the dashboards. For example:Ambar wrote:Is there a dashboard that tracks daily increase in cases and deaths by state for India similar to what we have for US ? The Ministry of Health dashboard just gives total numbers without day over day comparison. The last 24 hrs have seen the highest daily rise in cases and deaths in India at 7293 and 190 deaths.
 and this
and this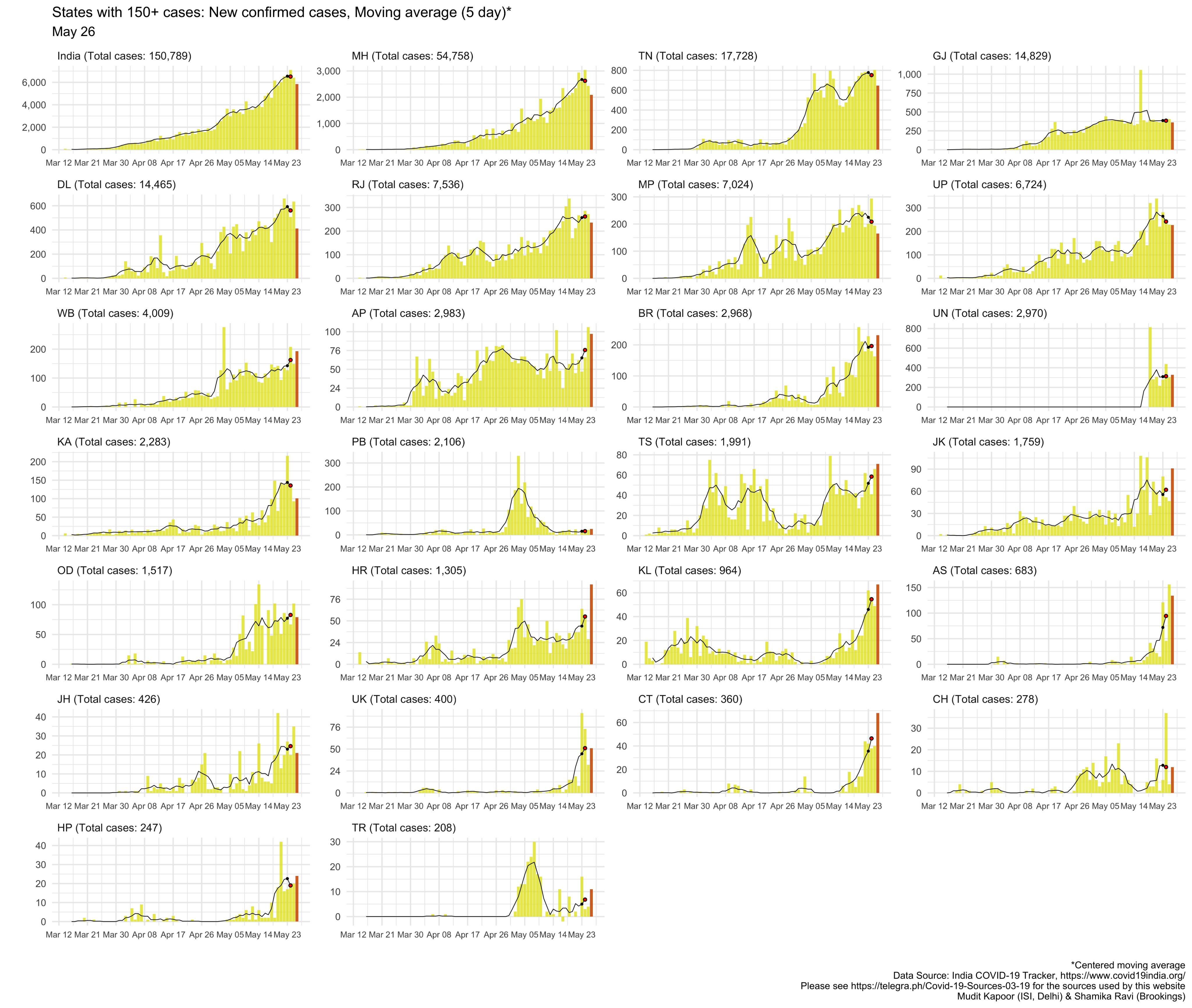
Hill, the Oxford scientist, has several arguments about why he thinks his vaccine is more promising than the others currently in human clinical trials.
First, he cites his team's many years of research on the technology used in their Covid vaccine.
The Oxford vaccine uses what's called an adenovirus vector. Adenoviruses cause the common cold, but in this case, the adenoviruses are weakened and modified to deliver genetic material that codes for a protein from the novel coronavirus. The body then produces that protein and, ideally, develops an immune response to it.
Hill and his colleagues have been working on adenovirus vaccines for nearly 20 years, and it's been used on thousands of study subjects in vaccines targeting more than 10 different diseases, according to the website for the Oxford vaccine.
Despite all this research, none of the Oxford vaccines has made it on the market, Hill said.
Still, Hill told CNN in the May 19 interview that his vaccine, plus one in China that also uses an adenovirus vector, are "the front runners" among the vaccines in clinical trials.
Hill then proceeded to disparage other teams' vaccines -- a highly unusual and aggressive move.
The four US vaccine candidates use a different technology -- or vaccine "platform" -- than Oxford.
Two of them, Moderna and Pfizer, use RNA vaccines, which inject a piece of genetic material from the novel coronavirus into human cells to stimulate immunity.
Hill described RNA vaccines as merely "noise from the new boys."
A Harvard University blog describes it differently.
"Compared to previous vaccines, this method is more robust, more versatile, and yet, equally efficient," according to the blog, which notes that the Bill & Melinda Gates Foundation invested $53 million in a German biotech company that specializes in RNA vaccines.
Hill was particularly disparaging of Moderna, which he said has "weird and wonderful technology." When asked what he meant by "wonderful," Hill said, "I was being sarcastic."
"They've got an unproven technology," he said.
CNN asked Moderna for its response, as well as Pfizer.
"Our only competitors in this race are the virus and the clock. We are rooting for multiple vaccines to succeed because we believe no manufacturer can make enough doses for the planet," according to the Moderna statement.
In March, Pfizer CEO Dr. Albert Bourla put out a five-point plan for companies to "work as one team across the industry."
"Our industry peers, the other pharmaceutical and biotechnology companies as well as health authorities, have come together like never before. We're acutely aware that we are all on the same side, and COVID-19 and other diseases are the enemy," Pfizer spokeswoman Amy Rose wrote in an email to CNN.
Hill also took a jab at Inovio, a US vaccine maker in clinical trials, saying "they can't scale up to get into phase three," clinical trials.
To take on the coronavirus, US vaccine makers consider an unprecedented strategy: working together
To take on the coronavirus, US vaccine makers consider an unprecedented strategy: working together
Inovio's technology uses a brief electrical pulse to deliver plasmids, or small pieces of genetic information, into human cells. Inovio says those cells then produce the vaccine, which leads to an immune response.
Jeff Richardson, a spokesman for the company said that "our competition is the virus, not other companies. There needs to be three or four winners to vaccinate the world. Most likely, there will be a number of vaccines that make it, and that's a good thing."
As for the four Chinese companies in clinical trials with a potential Covid vaccine, Hill said "they have a problem."
For a vaccine clinical trial to be successful, there needs to be sufficiently high levels of the virus circulating in the community. If there isn't enough virus around, it will be impossible to tell if the vaccine protected the study subjects, or if they were just never exposed to the virus.
"There's no Covid left in China. They can't finish," Hill said.
There is still a bit of Covid left in China, with a few dozen cases left, according to the latest briefings by the nation's National Health Commission. While this is likely not enough for a full-scale clinical trial, the researchers could conduct trials in other countries where the vaccine is still circulating more widely.
The Chinese vaccine is the farthest along, the only one that has published peer-reviewed phase I results. It uses a very similar technique as Oxford, using an Adenovirus as the vector. Since it uses a live virus that's well known to cause the common cold as the vector, its most common side effect is those of a common cold, which I imagine will be very similar with the Oxford vaccine.IndraD wrote:at the moment there are 240 vaccines in pipeline, each at different stage of development. Two of them quite talked about at the moment are: Oxford & Moderna. Oxford is into second phase of trial and Moderna will now recruit for phase I trial , they had promising results in Macac monkeys. Oxford one prevented pneumonia in monkeys but couldn't prevent infection.nam wrote:Some good news on vaccine development.
6 prototype DNA vaccine developed, showed remarkable efficiency in monkeys. All 6 developed immune protein and none of the monkeys had lung infection post vaccination. The ones which were not vaccinated, developed lung infection.
Higher the level of immunity protein, lower was the viral load.
https://www.bidmc.org/about-bidmc/news/ ... 19-vaccine
India has 14 vaccines in development process.
http://timesofindia.indiatimes.com/arti ... aign=cppst
they yet have to enter a trial phase but hope to develop a vaccine within 12 months, WHO has enlisted 3 vaccine companies of India.
It is surprising China in spite of harbouring virus since August hasn't developed a vaccine yet! Their vaccine Ad5-nCoV is a genetically engineered vaccine candidate with the replication-defective adenovirus type 5 as the vector to express SARS-CoV-2 spike protein, which intends to be used to prevent the disease caused by Covid-19. Phase I trial saw 108 inoculated with vaccine , while they produced cell & antibody mediated immunity, every patient developed fever, headache, chills
Such is the pressure on the govt, US has paid $1 billion to Astra Zenca co partnering Oxford vaccine in advance for 100 million shots. UK already is producing 30 million vaccine along with trial as vulnerable must be vaccinated before winter. From buzz US wants to give both (Moderna & Oxford) shots to its people.
Actually, if you read the Remdesivir trial closely, you'll see that not only is there a pretty strong trend toward improving mortality among all patients, a subgroup analysis would show that the lack of efficacy is primarily in the sickest groups. Take a look at figure 2 in the Remdesivir article. It's not unreasonable to think that if the medication is started early, it could prevent quite a few deaths.IndraD wrote:HCQ continues to be smeared, Gielad making kill though there is no evidence its drug Remdesivir works against nCV , a large librandu lobby in medical journal and msm are spearheading smear campaign against HCQ cos Trump stood by it.
WHO stops HCQ trials https://www.theguardian.com/world/2020/ ... fety-fears
Remdesivir approved in UK despite lack of evidence https://www.bbc.co.uk/news/health-52805828
France bans use of HCQ https://www.nytimes.com/2020/05/27/worl ... nk-af3ff1d


Of course! You had like 2 or 3 months headstart over others? That shows.DavidD wrote:
The Chinese vaccine is the farthest along, the only one that has published peer-reviewed phase I results.
Are you a virologist experienced in vaccine development to comment confidently as above? I would believe that the body to develop antibodies requires a spike protein from coronavirus. And this spike protein will cause similar side effects as that of the WuhanVirus as the body defenses start building up....Since it uses a live virus that's well known to cause the common cold as the vector, its most common side effect is those of a common cold, which I imagine will be very similar with the Oxford vaccine...
Maybe by accident an attenuated WuhanVirus was injected. How do you know that was not the case? Do you trust the data provided by the Communist dictatorship in China?I don't know enough about virology and immunology to know the difference between the COVID spike protein inserted into the Adenovirus by each team. It's interesting though that 6/6 vaccinated monkeys in animal studies with the Oxford vaccine contracted COVID (compared to 3/3 unvaccinated monkeys), but only 1/8 of ferrets with the Chinese vaccine contracted it (compared to 7/8 in unvaccinated ferrets).
But there is enough scientific data on the Chinese vaccine for WuhanVirus!I'm not trying to rag on HCQ, I suspect it can show an efficacy when used early as well, but there's simply no scientifically significant data to prove that thus far.
many thanks, very interesting read! While most of the allegations are based on assumptions and what ifs...it sounds resoundingly correct! I will post it in fullmukkan wrote:Anti-HCQ Paper in The Lancet Uses Fake Data
https://defyccc.com/anti-hcq-paper-in-t ... ated-data/
Good news: Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis, Harvey A Risch, May 27, 2020, American Journal of Epidemiology
Five studies, including two controlled clinical trials, have demonstrated significant major outpatient treatment efficacy. Hydroxychloroquine+azithromycin has been used as standard-of-care in more than 300,000 older adults with multicomorbidities, with estimated proportion diagnosed with cardiac arrhythmias attributable to the medications 47/100,000 users … These medications need to be widely available and promoted immediately for physicians to prescribe.
The rest of the post discusses a fraudulent paper (Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis, Mehra et al., May 22, 2020, The Lancet), based on Surgisphere’s fake data.
Surgisphere Corporation is fraud. Some of its services are being used for COVID-19 diagnostics and/or clinical decisions: QuartzClinical, COVID-19 Decision Support Tools (Rapid Triage, Severity Scoring, Diagnosis, Mortality Risk), Surgical Outcomes Collaborative analytics platform.
This anti-HCQ Lancet paper claims: “We included all patients hospitalised between Dec 20, 2019, and April 14, 2020, at hospitals participating in the registry” with COVID-19 and records of death or discharge. 671 hospitals from six continents allegedly provided their data for this database. The US hospitals were allegedly “selected to match the epidemiological characteristics of the US population”. In this great database, the authors have found 96,032 hospitalized patients with COVID-19, including 14,888 treated with HCQ or CQ with either Azithromycin (AZM) or Clarithromycin.
At this point, one would ask: Who has a database with detailed information on 96,000 patients? What about privacy laws? What about network security and data compatibility? Is Surgisphere a three-letter agency with such unrestricted global access? No, this is a one-man fraud. Paper’s second author is the man. There is no patients’ database and any raw data was fabricated or did not exist at all.
Some of the many mysteries in the alleged data:
The population data is very homogeneous, almost the same across the six continents – this is highly unlikely. For example, the patients with qSOFA < 1 are 82.6% in Europe and North America (what a coincidence!), and 82.7% in South America (Table S3).
The study data shows only 42% of patients (6,221 out of 14,888) in the treatment groups received HCQ+”macrolide”. However, it is widely known that almost all people treated with HCQ or CQ received HCQ+AZM.
In the study data for North America, 35% of patients (3,415) received CQ and 65% of patients (6,462) received HCQ. However, it is widely known that, outside of China, CQ was used significantly less than HCQ. The actual ratio is closer to 1:99 than to 35:65.
All patients treated with CQ/HCQ received antibiotics, almost always AZM. In the study, 32% of the North American patients received no “microlide” (Appendix, Table S3). Do peer reviewers really believe that almost a third of patients hospitalized with respiratory infection received immunosuppressives without antibiotics?
Tweets from an Australian MD & PhD helped explain some of the mystery. Not only is the data fake, but the database itself, Surgical Outcomes Collaborative, is fake as well. Here are some of the many red flags:
… the study says that they received data from 600 hospitals up to mid-April, and it was published end-May. This is impossible. If you have ever collected clinical research data you will know how impossible it is.
… you need ethical approval, suitable web servers set up, access set up for each hospital (and ideally each worker). This is what the authors say happened – they say they questioned existing repositories
I’m sorry but these don’t exist. Repositories do exist that maintain some of this data but it is mostly unlinked, relies on it being filled in correctly and exists on different systems that don’t talk to each other. …
… for each hospital system providing access you have to go through a complex ethics approval system (otherwise your hospital has just given your personal data to some strangers). …
An ethics approval of this magnitude would take months but it would take even longer to get the system up and running to allow secure access to hospital data systems externally. IT JUST DOES NOT HAPPEN.
The thread continues. The doctor takes note that the patients’ data matches, almost perfectly, on 23 factors.
This is pretty much statistically impossible, and they are claiming that they got this matching in 7000 patients out of a pool of 96000 patients for which they received high quality information from 671 hospitals. Nope. Sorry, the data is too “clean”.
And
So, this group are telling us that, not only have they got access to 671 hospital clinical record systems but (and this is a problem) … They have access to all the pathology providers linked to those 671 hospitals and THEY CAN DATA LINK THE PATIENT RECORDS. Not a chance.
This didn’t happen, and if it did it would be a massive privacy scandal. There would be serious financial and potentially criminal repercussions
So, either this data is completely fabricated -or- The authors have been able to overcome impossible hurdles of data sharing and probably broken a number of laws in the process.
Surgisphere has only a few employees, hired since February 2020. They did not overcome any hurdles.
This Lancet paper also heavily promotes Surgical Outcomes Collaborative, the fake database from which the data has been obtained. The “Collaborative” is owned by the Surgisphere Corporation. Sapan S. Desai, one of the paper’s authors, is a Surgisphere founder (disclosed) and CEO (not disclosed). The “Collaborative”, which was unknown before May 2020, is mentioned by name seven times in the paper. Two paragraphs (~500 words) are used as Collaborative advertisement: “a cloud-based health-care data analytics platform that includes specific modules for data acquisition, data warehousing, data analytics … A manual data entry process is used for quality assurance and validation to ensure that key missing values are kept to a minimum … ensures compliance with the US Food and Drug Administration (FDA) guidance on real-world evidence … data are collected through automated data transfers that capture 100% of the data from each health-care entity at regular, predetermined intervals … the standard operating procedures in place for each of the four ISO 9001:2015 and ISO 27001:2013 certified features of the registry … Collection of a 100% sample from each health-care entity is validated against financial records … capturing all-comer data and consecutive patient enrolment by capturing 100% of the data within electronic systems … compliant with the US Agency for Healthcare Research and Quality guidelines for registries …”.
None of this is true.
Surgisphere Corporation
Surgisphere’s website does not display its directors or executives, other than its founder-CEO Sapan S Desai (SSD) (About)
The website surgisphere.com is excluded from Archive.org – a sure sign that the company does not want people to know what it looked like in the past.
SSD worked in Surgisphere only part-time until February 2020, according to his profile in LinkedIn. His last full-time position was “Full-time clinical vascular surgeon, Medical Director of Surgical Quality, and Director of CME at Northwest Community Hospital” in Arlington Heights, IL
LinkedIn also shows profiles of four other employees. The three employees visible to me were hired in February 2020 or later. All of them live in different areas of the USA.
The address of Surgisphere is a private house in Palatine, IL, probably SSD’s home. (Buzzfile)
Surgisphere is not listed as a tenant at the address listed on its websites (875 N Michigan Ave, 31st Floor, Chicago, IL 60611)
In other words, Surgisphere Corporation existed mostly on paper, until February 2020, when its founder decided to cash out on the COVID-19 epidemic. Its software and services are vaporware.
The Surgical Outcomes Collaborative (database)
The Surgical Outcomes Collaborative has its own website surgicaloutcomes.com. Surgisphere markets it as an analytics platform, not a database. Nowhere does it claim possession of or access to patients’ information from multiple hospitals. Further, its website is a re-branding of another company’s website vascularoutcomes.com. Another Surgisphere’s website is quartzclinical.com.
Clarithromycin
This Lancet paper does not break down results between AZM and Clarithromycin, nor do they explain why they selected Clarithromycin, so I will. Clarithromycin has harmful interactions with CQ and HCQ (unlike AZM), and Clarithromycin is a strong QT prolonger, (unlike AZM, which is a mild one). This is why physicians did not use Clarithromycin with HCQ as a COVID-19 treatment. I could only find a couple of single patient reports of combining Clarithromycin with CQ. The authors included Clarithromycin in the paper to smear Azythromycin “by association.”
Remarks
This paper was published on Friday, May 22, on the eve of a long weekend. The paper immediately received wall to wall coverage in the media. On the same day The Lancet published a fawning comment Chloroquine or hydroxychloroquine for COVID-19: why might they be hazardous? These factors suggest careful coordination between the authors, the editors, and news media to catch the public and media community off-guard,causing maximum shock to reverberate over Memorial Dayweekend.
Surgisphere’s Rapid Triage, Severity Scoring, Diagnosis, Mortality Risk, advertised as artificial intelligence based tools for clinical decision support are nothing more than Javascript code, as simple as an online calculator. Additionally, this calculator is broken. Based on the company’s press releases, these tools have been covered by a range of media outlets from Yahoo! News to Breitbart. They were also covered in professional websites. Wow to those who relied on them. Unfortunately, they are easily accessible on the Surgisphere’s website.
Another Conflict of Interest?
The first author Mandeep R Mehra works in Brigham and Women’s Hospital, which conducts Gilead-initiated clinical trials of Gilead’s Remdesivir for COVID-19.
IndraD'ji, the nytimes is behind paywall and I have decided to not support racist tabloids like NYTimes. Sometimes they do put out good articles. But overall they are racist tabloids. The pink tabloids are better, at least they provide unpretentiously entertainment.IndraD wrote:https://www.nytimes.com/interactive/202 ... unity.html
far from herd immunity most of the people are still vulnerable to nCV , even in places with high death toll like NYC, only 20% infected so far.