Re: Wuhan Coronavirus Resource Thread
Posted: 16 Mar 2021 00:42
Consortium of Indian Defence Websites
https://forums.bharat-rakshak.com/
My observations:
The EU seems determined to make a complete mess of rolling out the vaccines against Covid-19. The latest bout of stupidity is the decision by several countries to suspend the use of the Oxford / AstraZeneca (AZ) vaccine.
...
Now, just to put the tin lid on this mess, several EU countries have decided to suspend use of the AZ vaccine in response to claims that it may be linked to an increased risk of blood clots. German health authorities have not covered themselves in glory on the issue. On Friday, health minister Jens Spahn said: ‘I regret that … some countries in the European Union have suspended vaccination with AstraZeneca. From what we know so far, the benefit… is far greater than the risk.’ By Monday, the government had decided to suspend use of the vaccine.
What is amazing is the paucity of evidence about an elevated risk of blood clots after taking the vaccine. It seems to be a classic example of the post hoc ergo propter hoc fallacy. Basically, just because B follows A does not mean that A caused B. In this case, Denmark was first to suspend the use of the AZ vaccine last week due to reports of clotting, including one fatal case. Other countries, including Norway and Iceland, quickly followed suit.
However, AstraZeneca has been quick to point out that there is no evidence of a problem. Yes, there have been cases of blood clots, but there don’t appear to be any more than one might expect. As David Spiegelhalter has pointed out, the European Medicines Agency says there have been 30 ‘thromboembolic events’ following around five million vaccinations. But, he notes, ‘Deep-vein thromboses (DVTs) happen to around one person per 1,000 each year, and probably more in the older population being vaccinated’.
Looking more broadly at the side effects from the vaccines, he concludes that ‘these vaccines have shown themselves to be extraordinarily safe’. He compares the logic to the misplaced scares around MMR and autism, which have led to many parents rejecting another safe vaccine. Of course, we should monitor the data in case a risk does arise – which is why we have a reporting system for side effects – but the data so far do not justify such extreme action.
So what is with the madness? EU countries are almost all way behind the UK and the US in vaccinating their populations. Supplies are thin on the ground, with every dose urgently needed. Yet they have chosen to reject, at least temporarily, an effective and safe vaccine.
The clue is in an idea that has dominated EU policymaking for decades: the precautionary principle. This suggests that precautionary action should be considered ‘even before a causal link has been established by absolutely clear scientific evidence’. That’s fine as long as the action taken has no downsides. But it’s mad when there is a clear and present danger to taking that action. In this case, refusing to use one kind of vaccine would be fine if there were plentiful supplies of alternatives or no health emergency. But evidence of the vaccine’s harm is still thin, bordering on non-existent, while Covid-19 is still killing far too many people.
Even if this suspension comes to an end and the AZ vaccine comes back into use, it will be no surprise if many people are hesitant about accepting it. The whole vaccination programme will be slowed down, more people will die and lockdowns will remain in place for longer. It’s hard not to wonder if there is a political element to all this – an attempt in some quarters to justify the EU’s vaccine failures by finding fault with a vaccine that was developed elsewhere. But even on the face of it, the decision to stop using the AZ vaccine is nuts.
Look at the growth rate of the cases, not the absolute number. The cases are widely dispersed across different states, and growing at about 3% every day. In about 25 days, the number of cases will double from the current 25000 a day and in another 25, they will double again. You are welcome to check the number of reported cases three weeks ago and see that doubling in three - four weeks is what the virus has been doing since it stopped declining in late Feb. Its worth recalling that about the same time last year, we had only a few hundred cases a day in India, which ramped up to 100,000 a day despite severe lockdowns. So its reasonable to expect similar growth through the summer months this year.. Nothing fundamental has changed. The only unknown here is the amount of exposure last year and existing immunity from prior infection. The rapid growth rate of the reported infections this year suggests that any existing immunity is not sufficient to prevent massive transmission.disha wrote:...
2. Overall case loads are dropping from the Sept/Oct peak. That is, in India the overall cases is @1/4th the peak
...
My relative traveled to MAA last week due to death in family, so was exempt from quarantine. I have enclosed current requirements (wef Feb. 22) below. Home quarantine/self monitoring for 14 days (see pdf for details). Take RT-PCR tests and upload 72 hours before flight. Seems you also need to register with TN govt. (enclosed).mappunni wrote:Saars Travel to India question.
A side question on travelers from the US to Chennai, do we have to undergo compulsory quarantine? I got both my vaccines and it's been a couple of weeks since I got the second shot. I received a lot of conflicting information from the travel agent who told me about compulsory quarantine for international travelers to Chennai. Thank you!
What a rip off by Chicom. $75 for something that doesn't produce enough anti-bodies. GOTUS pays $40 for 2 doses and GOI pays $4 for 2 doses.DUBAI—Health authorities in the United Arab Emirates have begun administering a third dose of the Sinopharm coronavirus vaccine to at least some residents, as doctors say the Chinese-made shots in some cases haven’t generated enough protective antibodies.
...
In a rare disclosure of the Chinese company’s commercial arrangements, Hungary said Thursday that it was paying about €63—or, $75— for every two doses of Sinopharm.
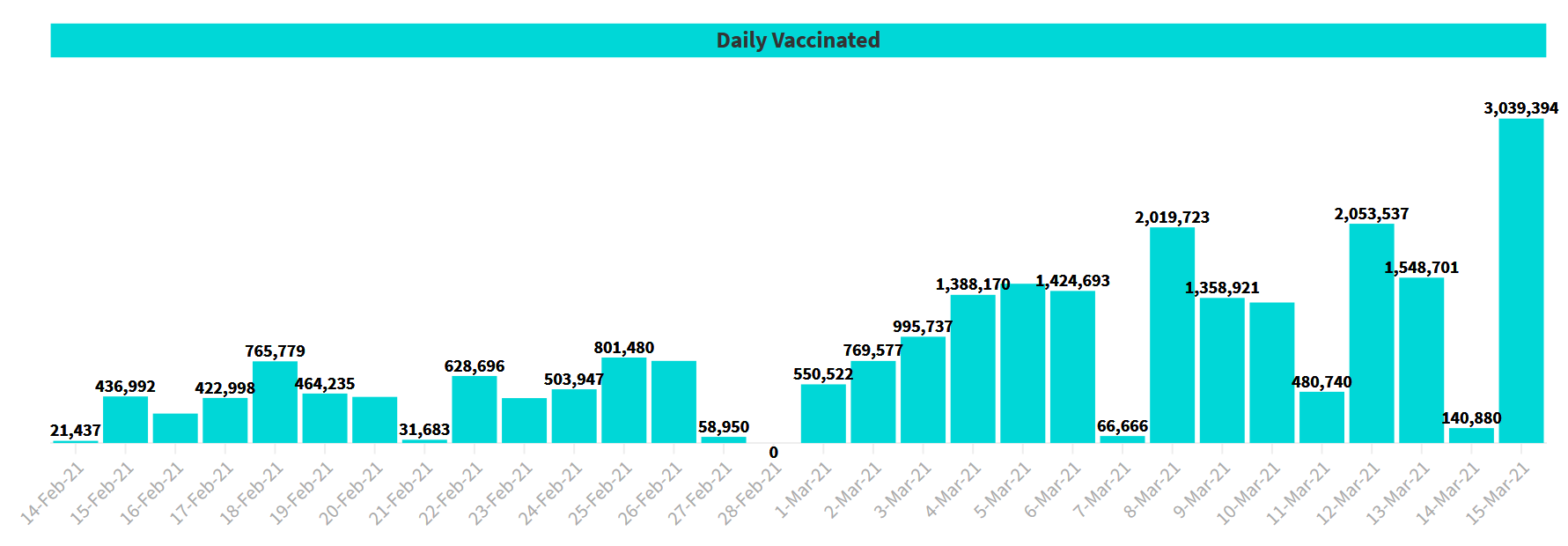
Put it this way, @2 crore people who could be in the crosspath of the virus and transmitting them further have been taken out of equation. That is means lesser number of hosts for the virus to infect, survive and transmit itself to others.A total of 30,39,394 vaccine doses were given on Monday, taking the total vaccinations in the country to 3,29,47,432 on the 59th day of the nationwide Covid-19 vaccination, as per the data shared by the ministry on its website.
Of these, 15,98,136 beneficiaries were administered the first dose and 2,65,487 healthcare and frontline workers received a second dose of vaccine as per the provisional report compiled till 7 p.m., it added.
More details behind paywall...The Centre has asked Maharashtra to scale up the pace of Covid-19 vaccination drive, especially in districts witnessing a sharp increase in cases.
Taking a grim view of the number of vaccine doses lying unutilised in the state, central health secretary Rajesh Bhushan has written to Maharashtra chief secretary Sitaram Kunte seeking his intervention to scale up vaccination.
...
US was doing 4M earlier so still some way to go. I have no doubt we will get there in a couple of weeks. Our next milestone is 80-90M vaccinated which is where US is atdhyana wrote:Impressive, India has surpassed US vaccination (daily) numbers.
US Vaccination numbers official tweet by WH
The 4-million dose was an incorrect number based on systemic data collating errors in CDC's tracking system. Daily Count of Total Doses Administered and Reported to the CDC by Date AdministeredTanaji wrote:US was doing 4M earlier so still some way to go.
One would be surprised to know how antiquated epidemiological surveillance tracking can be in the US. Much manual data entry/re-entry. And, so one gets errors like the above, with synchronization errors creeping in when trying to match up disparate datasets. Akin to the absolute mess brought about by authorizing numerous (dozens of) wholly incompatible electronic medical records systems- such that the venerable fax machine remains the most ubiquitous method of transferring patient data, generally speaking, amongst providers. This would be in stark contrast to almost every other industrialized country having a high penetration of (and/or mandated ) EMR's which mostly talk to each other, usually because it's the same EMR used throughout said country. The US' heterogeneous healthcare system has great strengths and great weaknesses.Due to a delay in data syncing on March 13, 2021, 4,575,496 new doses administered were initially reported, which included records that were reported after 6:00 AM ET (the regular cutoff time for daily reporting). The site has since been updated to reflect the totals reported as of March 13 at 6:00 AM ET. Totals for March 14, 2021 reflect the number of doses reported through the regular daily reporting period.
That’s a very impressive increase. But Yeh Dil Mange More!!disha wrote:https://swarajyamag.com/insta/india-cro ... h-ministry
Major milestone crossed! 30 lakh vaccinations in one day and 3 crore vaccinations so far.
 via@manoj_kotak
via@manoj_kotakROME (Reuters) - The decision by Germany, France and Italy to suspend AstraZeneca’s COVID-19 shots after several countries reported possible serious side-effects is a “political one”, the director general of Italy’s medicines authority AIFA said on Tuesday.
“We got to the point of a suspension because several European countries, including Germany and France, preferred to interrupt vaccinations... to put them on hold in order to carry out checks. The choice is a political one,” Nicola Magrini told daily la Repubblica in an interview.
Magrini said that the AstraZeneca vaccine was safe and that the benefit to risk ratio of the jab is “widely positive”. There have been eight deaths and four cases of serious side-effects following vaccinations in Italy, he added.
Aifa will take two to three days to collect all required data and once “doubts are cleared we can carry on at a faster speed than before,” Magrini said.
Health experts say they are disappointed and confused by the flurry of suspensions of the coronavirus vaccine developed by AstraZeneca and the University of Oxford, warning there is not yet enough data to justify these decisions.
The EU regulatory body is "fully convinced" that the vaccine's benefits outweigh possible risks. Global health experts have been under growing pressure to answer questions over the safety of AstraZeneca's COVID-19 shot.
There is "no indication'' that AstraZeneca vaccines are the cause of blood clots reported in some shot recipients, the European Medicines Agency's (EMA) chief said on Tuesday.
The regulatory agency responded after more than a dozen EU countries suspended the Oxford-AstraZeneca coronavirus vaccine amid health concerns.
The agency is "still firmly convinced that the benefits of the AstraZeneca vaccine in preventing COVID-19 with its associated risk of hospitalization and death outweigh the risk of these side effects," Executive Director Emer Cooke added.
Cooke said that an EMA evaluation of individual incidents is ongoing. It is expected to complete a full review on Thursday.
Excellent. Hope we can go to 5 million/daySuraj wrote:1.92 million vaccinations up to 7pm for Tuesday . Yesterday when it crossed 3 million for the full day, it was 1.86 million at 7pm . Seems they can cross 3 million again today.
As said before, there is a scam going on of enormous proportion. Lots of money involved for some of the big pharma companies. I wouldn't be surprised if various EU members have been bribed. The EU has fallen behind in vaccinations, and I would speculate that India will catch up to the EU by the end of this month.
10M/day is unlikely, but 4-5M/day is. Sunday is a big slow down in government hospitals and clinics.Raja wrote:5 million will happen and probably soon. 10M/day would be awesome
India is already the first to get to 3 million a day . The US was the first to 1 and 2 million marks .vijayk wrote:Excellent. Hope we can go to 5 million/daySuraj wrote:1.92 million vaccinations up to 7pm for Tuesday . Yesterday when it crossed 3 million for the full day, it was 1.86 million at 7pm . Seems they can cross 3 million again today.

I am not an immunology expert. Perhaps, somebody from the forum can chime in. I have not heard any negative about UK's experience with the delayed second dose.vera_k wrote:If that is the case, is it advisable to delay the second dose of the vaccines? 700 million people could be vaccinated in the same time or 347 million in 2.5 months.
These numbers are optimistic since the known production capacity (70m/month + 12m/month) posted earlier in the thread is enough to support about 2.8M vaccinations per day. I haven't found any sources indicating plans to import vaccines, so these are likely all that's available for now.
By the 3rd week of January, about 1 million vaccinations/day and Operation Warp Speed had envisioned some 660 million cumulative doses within 6 months after the start on 17 December 2020.V_Raman wrote:I am amazed at the speed of vaccinations in USA - they have caught up finally after all the tragedies. They will soon start exporting the vaccines.
EXCLUSIVE-Ocugen plans to sell 100 mln Indian vaccine doses in U.S. in 2021
NEW DELHI, March 15 (Reuters) - Ocugen Inc plans to sell 100 million doses of India’s state-backed COVID-19 vaccine in the United States this year, the U.S. firm’s chief executive Shankar Musunuri told Reuters on Monday.
Musunuri said Ocugen, a Pennsylvania-based biopharmaceutical firm, was aiming to launch the Indian-developed vaccine in the United States in the second quarter of 2021, initially with imported shots before beginning production there.
The United States has already authorised COVID-19 vaccines developed by Pfizer/BioNTech, Moderna and Johnson & Johnson for emergency use.
India’s two-dose COVAXIN has been found to be 81% effective in an interim analysis of late-stage trial data on some 26,000 people in India, its developers Bharat Biotech and the state-run Indian Council of Medical Research said this month.
Bharat Biotech says as many as 40 countries are interested in COVAXIN and it has already sought emergency approvals in Brazil and the Philippines. Cracking the U.S. market would be a significant milestone for the company and India’s vaccine industry, which is the world’s largest.
Musunuri said Ocugen had held initial talks with the U.S. Food and Drug Administration and planned to seek emergency use authorisation in April.
“They’re fine with the way the interim analysis is being done,” Musunuri said, adding that Ocugen had “a regulatory path” to take the process forward.
Musunuri said COVAXIN had the potential to work against COVID-19 variants and Ocugen could initially focus on children as it was likely to be safe for those over the age of 12, while shots produced by other drugmakers targeted adults.
Pfizer’s vaccine has been authorised for emergency use in the United States for individuals 16 years and older, with the other two given authorisation for emergency use in people 18 years and above.
“Like a polio virus given to babies, this could be safe for all children, high-risk groups, pregnant women and people with comorbidities,” Musunuri said of COVAXIN.
BOOSTER SHOT
He said Ocugen was expecting more data from Bharat Biotech on COVAXIN, following up on a study by the Indian company in January which showed the shot was likely to be effective against the British strain of the coronavirus.
India’s drug regulator approved COVAXIN for emergency use in January for people above 12, saying it could act against the whole body of a virus instead of just its “spike-protein” tip, potentially making it more effective in case of mutations.
Musunuri said this could mean it could be used as a booster for people already vaccinated with a first dose of other shots.
He said Bharat Biotech would export “tens of millions of doses” to Ocugen, which is also finalising U.S. contract- manufacturers for the product.
Ocugen will have the U.S. rights to the vaccine and will be responsible for its clinical development, regulatory approval and commercialisation there. Bharat Biotech will also transfer its technology, and keep 55% of the profit.
Shares in Ocugen have nearly tripled this year, taking it close to a $2 billion market capitalisation, helped by a spike when Bharat Biotech announced its late-stage trial results.
COVAXIN has been given to more than 2 million prioritised adults in India since the country’s vaccination drive began in mid-January. Bharat Biotech says it wants to produce about 700 million doses a year domestically.